A kit for isothermal detection of bacterial pathogens in respiratory tract infections with enzyme-cleavage probes
A technology of pathogens and respiratory tract, which is applied in the field of reagent kits for the constant temperature detection of bacterial pathogens of respiratory tract infection with enzyme-cutting probes, which can solve the problems of difficulty in achieving sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
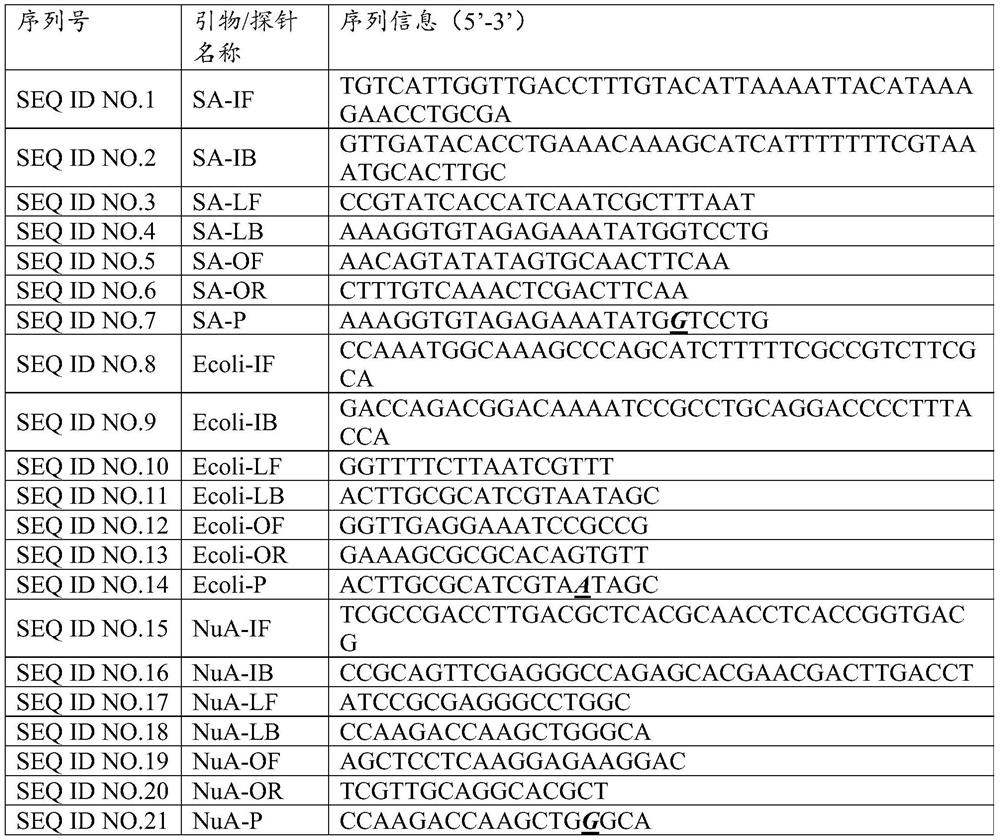
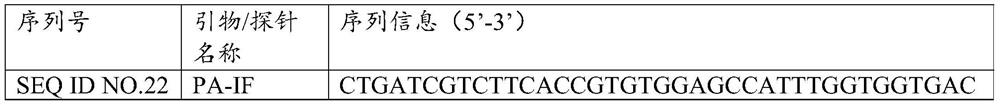
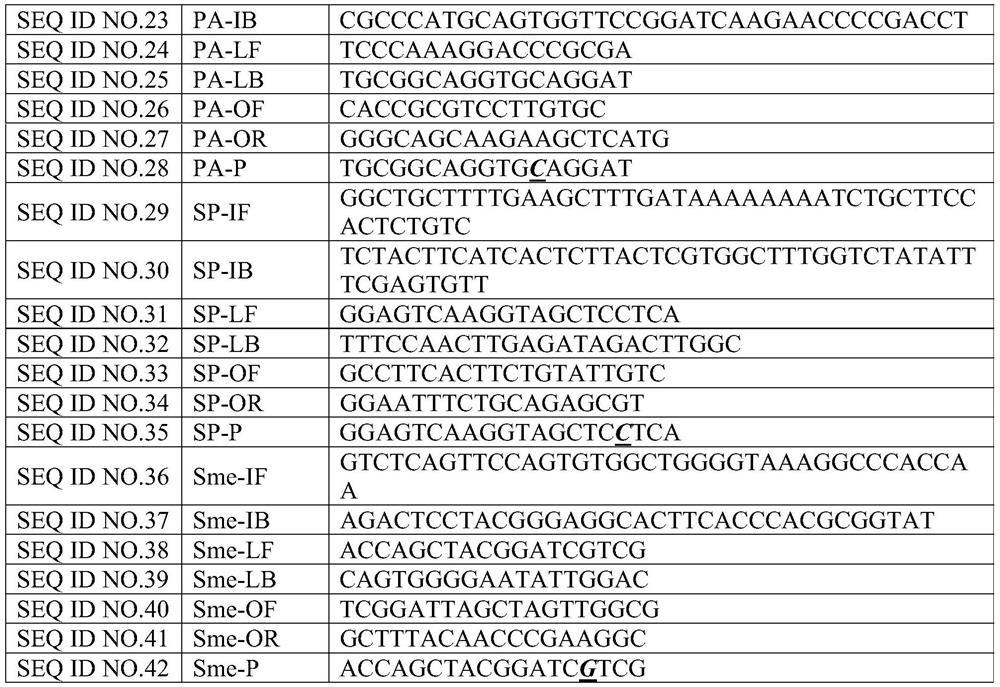
[0027] Example 1: Determination of the primer probe combination of 12 bacterial disease primary nucleic acid detection of 12 bacterial disease
[0028] 1. Design of single -weight primers and probes
[0029] The present invention has against Golden Polyris (SA), Copper Green Pseudomona (PA), Balman Bacteria (ABA), Pneumonia Crastacilla (KPN), Bachalia (Hi), Purakami Legion (LP), bowel gallery (EC), pneumonia, pneumococcal (SP), malt narrow -eating fake monolm (SME), onion Berk Holde (BC), E. E. E. Cororneya (ECOLI), Star -shaped slaves Bacterial pathogenic gene sequences such as Capillary (NUA) are analyzed. Based on the basic principles of the primer probe design, the design software is used for designing. The combination of the three sets of primers is shown in Table 1.
[0030] Table 1:12 Sequence information of the ranging of bacterial pathogenic programs of the primer
[0031]
[0032]
[0033]
[0034]
[0035]
[0036] 2. Screening of single -weight primers and pr...
Embodiment 2
[0059] Example II: Verification of the testing of the test kit and the sensitivity and specificity of the kit in the present invention
[0060] 1. Preparation kit
[0061] Four nucleic acid reaction liquids, blank control products, and positive control products are packed together, and the product uses instructions are accompanied by the instructions of the enzyme cutting probe in the present invention. Among them, the composition of nucleic acid reaction solution, blank control and positive control products is shown in Table 6 below to detect the fluorescent group marks of the probe of each pathogen.
[0062] Table 6. The ingredients composition of the kit
[0063]
[0064] Table 7. Each probe in the kit The fluorescent base group mark instructions
[0065]
[0066]
[0067] 2. Test 10 cases of clinical sample DNA in the kit in the present invention
[0068] 2.1 The DNA information of the 10 clinical samples used in the test is shown in Table 8 below.
[0069] Table 8. Test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com