Synthetic method for the preparation of an hydrazine compound
A technology for compounds and complexes, applied in the preparation of hydrazine, organic chemistry, etc., can solve problems such as providing effective methods that have not yet been met
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] The method is described with respect to the introduced amount of compound 1 (1.0 eq).
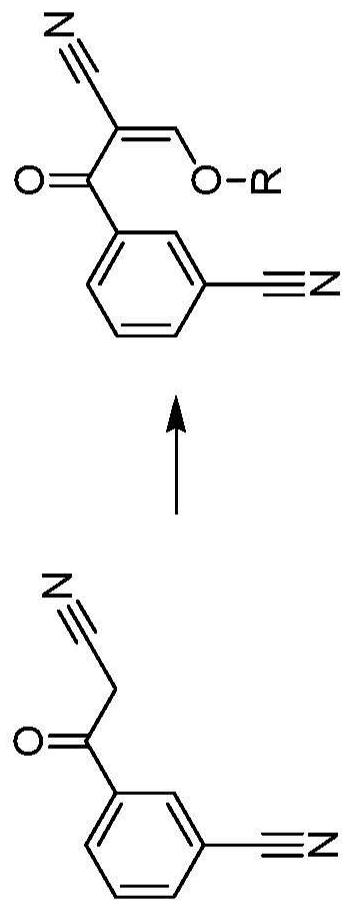
[0177] Acetic anhydride (2.0 eq) was added to a mixture of 3-(cyanoacetyl)benzonitrile (Compound 1) (1.0 eq) in toluene (4.5 vol) and heated to 105°C. Slowly reduce the pressure to 800-900mbar until a slight reflux is obtained. Triethyl orthoformate (1.5 eq) was added during the distillation. The reaction was then stirred for 1 h. The reaction was cooled to 25°C and the product crystallized. n-Heptane (5 vol) was added within 0.5 h. The reaction was further cooled to less than 5 °C and stirred for 1 h. The product was filtered off, washed with n-heptane (2 x 2 vol) and dried under vacuum. 3-[2-Cyano-2-(ethoxymethylene)acetyl]benzonitrile (compound 2) was obtained as a red solid in 90% yield and 98% purity (HPLC, area %).
Embodiment 2
[0179] The method is described with respect to the introduced amount of compound 4 (1.0 eq).
[0180] Hydrochloric acid (30%, 3.7 eq) was added to a suspension of 3-amino-4-methylbenzoic acid (compound 4) (1.0 eq) in water (1.5 vol). The suspension was cooled to less than 10°C and stirred for 0.5 hours. A solution of sodium nitrite (1.1 eq) in water (0.85 vol) was added slowly while maintaining the temperature below 10°C. The reaction was then stirred for 0.5 hours. A cooled (<5°C) suspension of sodium sulfite (4.9 eq) in water (10 vol) was added to the reaction mixture while maintaining the temperature below 15°C. The reaction was then stirred for 1 hour. The resulting mixture was heated to 60 °C for 1 h. Hydrochloric acid (30%, 7.2eq) was added, and the mixture was stirred at 80°C for 2 hours. The mixture was cooled to 25°C. Aqueous sodium hydroxide solution (33%) was added (final pH: 5.6-5.8). The mixture was then stirred for 1 hour, filtered, washed with water (2×4 ...
Embodiment 3
[0183] The method is described relative to the introduced amount of Compound 2 (1.0 eq).
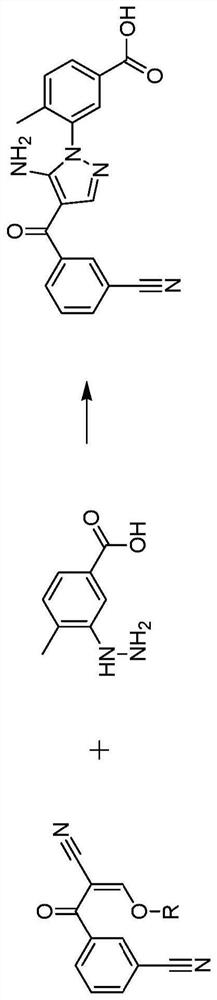
[0184] A solution of 3-[2-cyano-2-(ethoxymethylene)acetyl]benzonitrile (compound 2) (1.0 eq) in dimethyl sulfoxide (4 vol) was added at 105 °C over 1.5 h Into a solution of 3-hydrazino-4-methyl-benzoic acid (compound 3) (1.2 eq) in dimethyl sulfoxide (20 vol). The reaction was then stirred for 1 h. Water (24 vol) was added at 60°C within 1 hour and the mixture was then cooled to 20°C. The mixture was then stirred for 1 hour, filtered, washed with water (2 vol), washed with cold methanol (<5°C, 2 x 4 vol) and dried under vacuum. 3-[5-Amino-4-(3-cyanobenzoyl)-1H-pyrazol-1-yl]-4-methylbenzoic acid (compound 5) was obtained as pale yellow solid in 52% yield , purity 98% (HPLC, area %).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com