Method for inducing differentiation into dopamine neuronal precursor cells from stem cells
A technology of dopamine nerve and precursor cells, applied in biochemical equipment and methods, animal cells, nervous system diseases, etc., can solve problems such as stem cell differentiation that have not yet been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
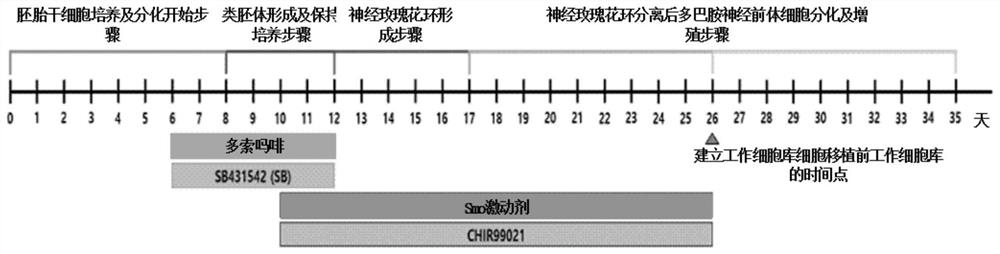
[0128] Example. Protocol for Differentiation into Dopamine Neural Precursor Cells
[0129] For the human embryonic stem cells or induced pluripotent stem cells cultured above, they were stabilized by 2 subcultures from the time point when the master cell bank (Master Cell Bank, MCB) was thawed, and differentiated dopaminergic precursors were started by using the third passage dish. somatic cells.
[0130] The date of preparing the third-generation culture dish was set as the beginning of differentiation (d0), and on the 6th day of differentiation (d6), human embryonic stem cell culture fluid (TeSR2, STEMCELL, SCR5860) was treated with 5uM doxomorphine (Millipore, 171260) and 5uM SB431542 (hereinafter referred to as SB) (Sigma, S4317) were pretreated for 2 days until the eighth day, thereby increasing the occupancy rate of differentiation into neuroectoderm.
[0131] On the eighth day of differentiation (d8), for human embryonic stem cells cultured in the form of a single cell...
experiment example 1
[0138] Experimental example 1. Stem cell culture steps
[0139] 1-1. Optimization of the treatment time of doxomorphine and SB431542
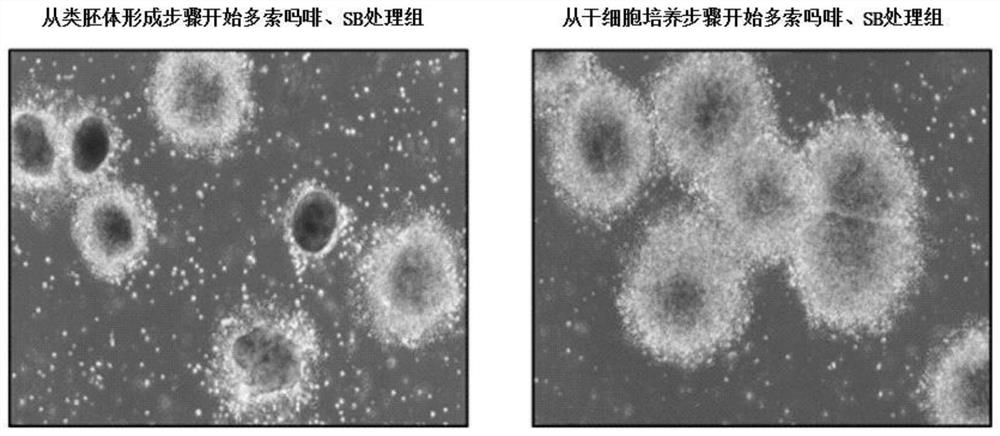
[0140] The method of treating doxomorphine and SB431542 from the 8th day of culture was used instead of the treatment of doxomorphine and SB431542 from the 6th day of culture, and compared with the method of the present invention.
[0141] From Figure 2a It can be confirmed that compared with the case of treating doxomorphine and SB431542 from the 8th day as the embryoid body formation step (the conventional method), it can be seen that the pretreatment from the 6th day of the stem cell culture step is more effective. The state and yield of the neural rosettes formed in the case of somorphine and SB431542 (the method of the present invention) were better.
[0142] These results suggest that not only the neuroectodermal differentiation occupancy can be increased but also the final dopamine can be greatly increased by pretreatment of doxomorph...
experiment example 2
[0146] Experimental example 2. Embryoid body formation and maintenance culture steps
[0147] 2-1. Optimization of culture time
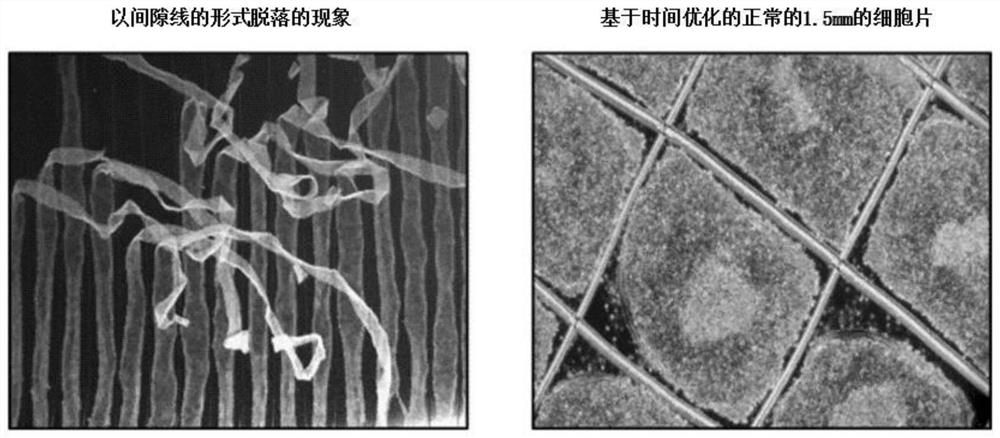
[0148] Instead of attaching the embryoid bodies to the Petri dish on culture day 12 (embryoid body formation / maintenance day 4), a method of treating doxomorphine and SB431542 and inducing embryoid bodies up to day 13 in culture was used, and Compare with the method of the present invention.
[0149] From image 3 It can be confirmed that until day 4 (day 12 in culture), most embryoid bodies are well kept alone, but after day 5 (day 13 in culture), the embryoid bodies adhere to each other with high frequency and the chunks appear again.
[0150] 2-2. Optimization of treatment time of Smo agonists and CHIR99021
[0151] The method of treating the Smo agonist and CHIR99021 from the 6th or 8th day of culture was used instead of the treatment of the Smo agonist and CHIR99021 from the 10th day of culture, and compared with the method of the present i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com