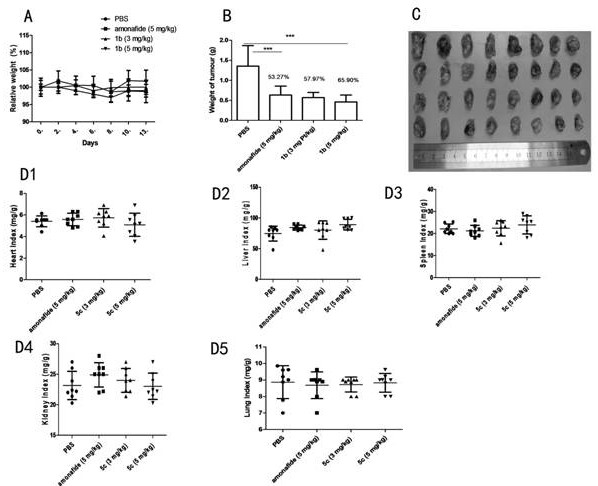
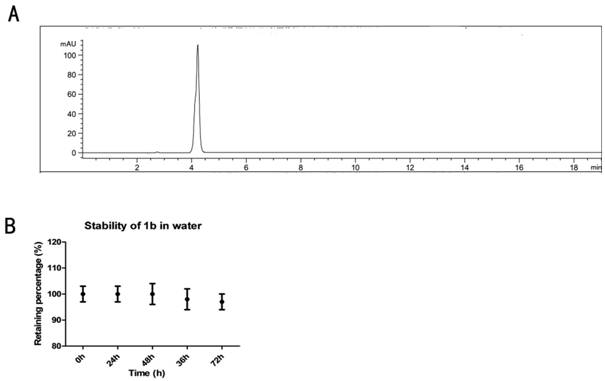
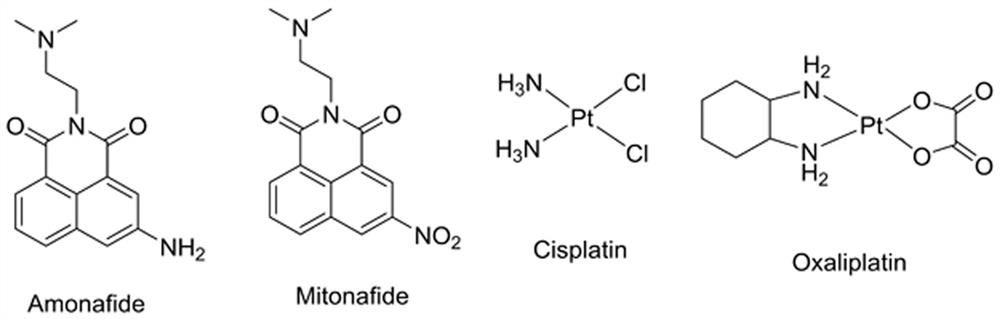
A kind of naphthalimide indole heterocyclic compound, its preparation method and application
A technology of naphthalimide indole and heterocyclic compound, which is applied in the field of medicinal chemistry, can solve the problems of limited application, inhibition, vomiting, venous inflammation, central system toxicity, etc., and achieves increased intake and maximum tolerated dose. , improve the effect of effective combination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: 1b
[0057]
[0058] 1 H NMR(300MHz,DMSO-d6)δ8.71(s,1H),8.24(s,1H),8.06(s,1H),7.75(s,1H),4.26(d,4H), 2.94(t, J=27.2Hz, 6H), 2.01(d, J=24.3Hz, 4H). 13 C NMR (75MHz, D 2 O)δ167.17, 164.14, 163.39, 135.27, 134.36, 134.00, 131.51, 130.29, 127.77, 123.66, 121.97, 120.24, 45.36, 44.65, 44.55, 43.07, 37ion.51, 24.163, I [M-H] + calcd 365.19, obsd 365.19. Its preparation method is as follows:
[0059] (1) Add 100mmol (19.8g) of compound 8 (1,8 naphthalene dicarboxylate) and 100mL of concentrated sulfuric acid into a 250mL volumetric flask, stir and dissolve in an ice bath, measure 11.2mL of concentrated nitric acid and 50mL of concentrated sulfuric acid to prepare a mixed acid , dropped into the concentrated sulfuric acid solution of compound 8 under ice-bath conditions, continued to react for 2.5h after the drop, after the reaction was completed, poured the reaction solution into ice water, let it stand, filtered with suction, washed with water until neutral...
Embodiment 2
[0065] Example 2: 2b
[0066]
[0067] 1 H NMR(300MHz,DMSO-d6)δ8.69(d,J=14.3Hz,2H),8.36(s,1H),7.82(s,1H),4.42(s,2H), 4.11(s,2H) ,3.11–2.70(m,6H),2.05(s,2H),1.79–1.41(s,4H). 13 C NMR (75MHz,D 2 O)δ 167.33,164.43,163.73,135.40,134.49,130.70,130.32,127.63,123.14,122.53,121.86,120.31, 119.78,46.99,45.24,43.07,38.74,37.35,24.18,23.90,22.77.ESI-MS(positive ion mode) m / z [M+HCl] + calcd 417.10, obsd 417.10.
[0068] The difference between the preparation method of compound 2b in Example 2 and Example 1 is that in step (4)) compound 12a (1 mmol) and polyamine chain (2mmol) and acid anhydride (3mmol) were subjected to condensation reaction for 24-48h to obtain compounds with or without Boc protecting groups, and those with protecting groups removed the Boc protecting groups to obtain compound 2b.
[0069] The 2b synthetic route reaction formula of the polyamine analog modified by naphthalimide indole core is:
[0070]
Embodiment 3
[0071] Example 3: 3b
[0072]
[0073] 1 H NMR (300MHz, DMSO-d6) δ10.66(s, J=14.3Hz, 1H), 8.36(s, 1H), 8.81(s, 1H), 7.97(s, 1H), 7.78(s, 1H) ,4.10(s,2H),4.07(s,2H),2.68(s,2H),2.53(m,8H),1.78(s,2H),1.74(s,2H),1.5(s,3H), 1.38(s,4H). 13 C NMR (75MHz,D 2O) δ176.6, 159.3, 159.3, 145.2, 132.0, 131.6, 129.5, 128.1, 124.3, 123.6, 123.0, 117.1, 116.4, 56.1, 49.6, 49.6, 46.0, 46.0, 39.4, 35.6, 31.2, 33.2, ESI-MS(positive ion mode)m / z[M] + calcd 437.24, obsd 437.24.
[0074] The difference between the preparation method of compound 3b in embodiment 3 and embodiment 1 is that in step (4), compound 12a (1mmol) and polyamine chain (2mmol) and acid anhydride (3mmol) were subjected to condensation reaction for 24-48h to obtain a compound with or without a Boc protecting group, and the compound 3b was obtained by removing the Boc protecting group with a protecting group.
[0075] The 3b synthetic route reaction formula of the polyamine analog modified by naphthalimide indole core is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com