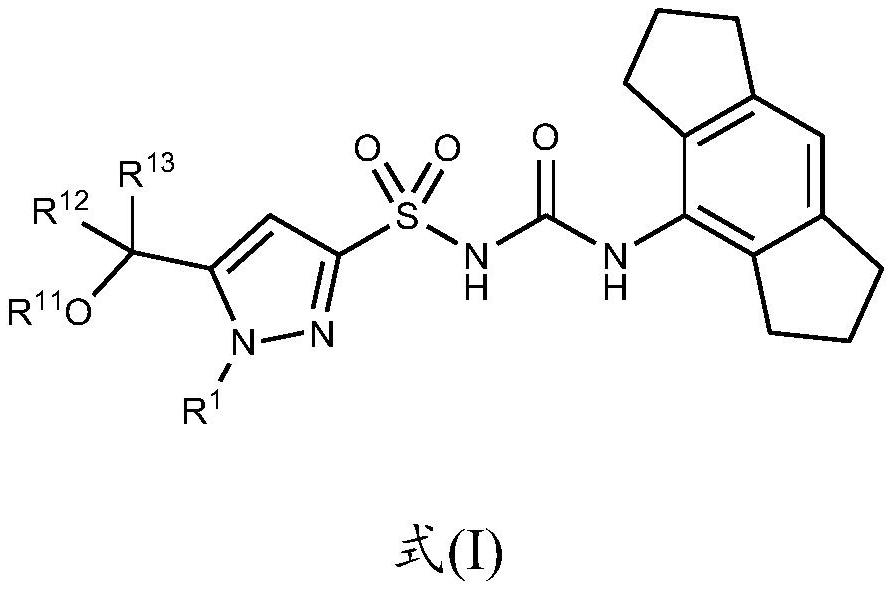
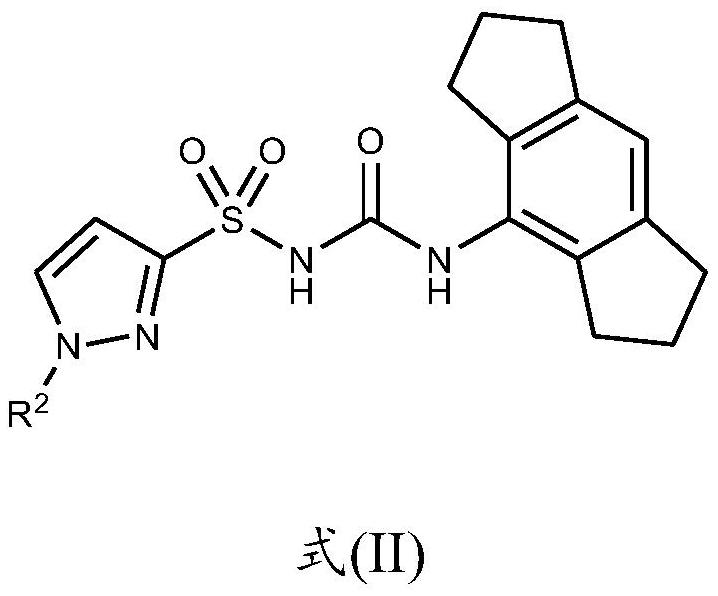
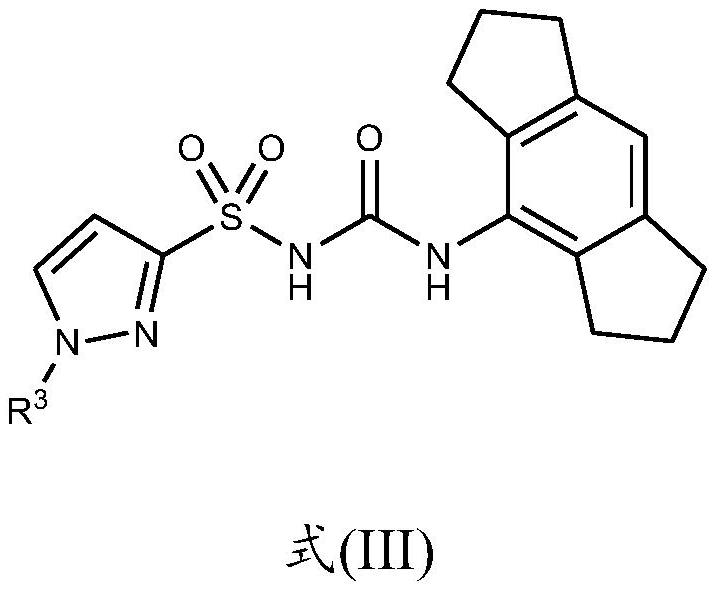
Nlrp3 inhibitors
A technology of R12, C1-C3, applied in the field of NLRP3 inhibitors, can solve the problem of limited efficacy and non-specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1253] Example 1: 5-fluoro-N-((4-(2-hydroxypropan-2-yl)-2-methylphenyl)sulfonyl)-7-isopropyl-3,3-dimethyl-2,3- Dihydro-1H-indene-1-carboxamide
[1254]
[1255] 4-(2-Hydroxypropan-2-yl)-2-methylbenzenesulfonamide (Intermediate L1) (36 mg, 0.16 mmol) and 5-fluoro-7-isopropyl-3,3-dimethyl -2,3-Dihydro-1H-indene-1-carboxylic acid (Intermediate R7, Step B) (39 mg, 0.16 mmol) was stirred in DCM (6 mL). EDC (60 mg, 0.31 mmol) and DMAP (38 mg, 0.31 mmol) were added to the reaction mixture. The mixture was stirred at room temperature overnight, diluted with DCM (5 mL), and washed with 1M aqueous HCl (3 mL). Concentrate the organic phase. The residue was dissolved in DMSO (0.5 mL), and KO was added t Bu (52 mg, 0.47 mmol). The mixture was purified by reverse phase preparative HPLC method 2 to afford the title compound (12 mg, 17%) as a white solid.
[1256] 1 H NMR (300MHz, methanol-d 4 )δ7.95(d,1H),7.36(s,1H),7.31(d,1H),6.69–6.60(m,1H),6.60–6.51(m,1H),3.96(s,1H),2.71 (s,...
Embodiment 2
[1258] Example 2: 2-(4-fluoro-2,6-diisopropylphenyl)-N-((4-(2-hydroxypropan-2-yl)-2-methylphenyl)sulfonyl)acetamide potassium salt
[1259]
[1260] 4-(2-Hydroxypropan-2-yl)-2-methylbenzenesulfonamide (Intermediate L1) (50 mg, 0.17 mmol) and 2-(4-fluoro-2,6-diisopropylphenyl ) Acetic acid (Intermediate R11) (42 mg, 0.17 mmol) was stirred in DCM (6 mL). EDC (67 mg, 0.35 mmol) and DMAP (43 mg, 0.35 mmol) were added to the reaction mixture. The mixture was stirred overnight, diluted with DCM (5 mL), and washed with 1M aq. HCl (mL). Concentrate the organic phase. The residue was dissolved in DMSO (0.5 mL), and KO was added t Bu (59 mg, 0.52 mmol). The mixture was purified by reverse phase preparative HPLC method 2 to afford the title compound (41 mg, 52%) as a white solid.
[1261] 1 H NMR (300MHz, methanol-d 4 )δ7.90(d,1H),7.34(d,1H),7.28(dd,1H),6.69(d,2H),3.64(s,2H),3.19–2.98(m,2H),2.68(s ,3H), 1.48(d,6H), 1.07(d,12H).
[1262] LCMS: m / z 450 (M+H) + (ES + );448(M...
Embodiment 3
[1263] Example 3: 3-(4-fluoro-2-isopropyl-6-(2-methoxypyridin-4-yl)phenyl)-N-((4-(2-hydroxypropan-2-yl)-2- Methylphenyl)sulfonyl)propionamide
[1264]
[1265] 4-(2-Hydroxypropan-2-yl)-2-methylbenzenesulfonamide (Intermediate L1) (85 mg, 0.37 mmol) and Et 3 N (38 mg, 0.37 mmol) was stirred in DCM (6 mL). Then 3-(4-fluoro-2-isopropyl-6-(2-methoxypyridin-4-yl)phenyl)propionyl chloride (Intermediate R5) (63 mg, 0.19 mmol) in DCM was added dropwise (1 mL) and stirred overnight. EDC (36 mg, 0.19 mmol) and DMAP (23 mg, 0.35 mmol) were added to the reaction mixture. The mixture was stirred overnight, diluted with DCM (5 mL), and washed with 1M aqueous HCl (3 mL). Concentrate the organic phase. The residue was dissolved in DMSO (0.5 mL) and purified by reverse phase preparative HPLC method 2 to afford the title compound (22 mg, 22%) as a white solid.
[1266] 1 H NMR (300MHz, methanol-d 4 )δ8.14–8.09(m,1H),7.87(d,1H),7.34(d,2H),7.10–7.00(m,1H),6.83(dd,1H),6.72–6.61(m,2H) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com