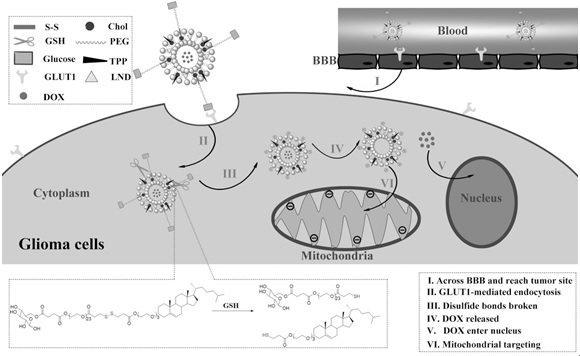
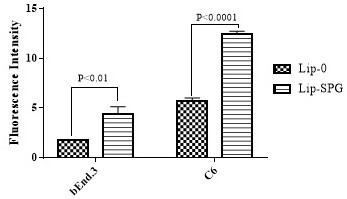
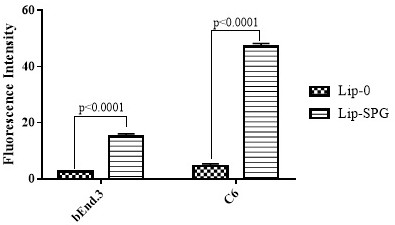
Preparation and Application of Brain Tumor Targeting Liposomes Modified by Glucose and Triphenylphosphonium
A technology targeting liposomes and triphenylphosphonium, applied in liposome delivery, anti-tumor drugs, medical preparations of non-active ingredients, etc., can solve problems such as increased toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of compound 3
[0029]
[0030] At -5 °C, 3,3'-dithiodipropionic acid (4.054 g, 19.28 mmol) CH 2 Cl 2 (30 mL) solution was added EDCI (4.75 g, 24.1 mmol), DMAP (2.95 g, 24.1 mmol), and DIPEA (3.74 g, 28.92 mmol), and stirred for 30 minutes. Then, CH 2 Cl 2 (30 mL) solution and react overnight at room temperature. The reaction solution was washed sequentially with aqueous HCl (1N, 100 mL×2) and saturated NaCl (100 mL×3). The organic phase was further treated with anhydrous Na 2 SO 4 Dry and remove solvent. Finally, by silica gel column chromatography (CH 2 Cl 2 / CH 3 OH=300 / 1) to obtain light yellow semi-solid (5.067 g), yield 74.06%. 1H-NMR (400 MHz, CDCl3, ppm) δ: 0.67 (s, 3H), 0.86 (dd, 6H, J 1 = 6.6Hz, J 2 = 1.6 Hz), 0.91 (d, 3H, J =6.4 Hz), 0.99 (s, 3H), 0.85-2.39 (remaining cholesterol protons), 2.75-2.79(m, 4H), 2.93 -2.97 (m, 4H), 3.15-3.24 (m, 1H), 3.64- 3.68 (m, 8H), 3.72-3.74(m, 2H), 4.26-4.28 (m, 2H), 5.34 (s, 1H). HRMS (ESI) ...
Embodiment 2
[0032] Preparation of Compound 4
[0033]
[0034] At -5 °C, CH 2 Cl 2 (10 mL) solution, add DCC (257 mg, 1.247 mmol) and DMAP (25 mg, 0.208 mmol) and stir for 30 minutes. Then, PEG (1.039 g, 1.04 mmol) in CH 2 Cl 2(10 mL) solution, stirred overnight at room temperature. After filtering off by-products (DCU), the reaction solution was concentrated. Finally, by silica gel column chromatography (CH 2 Cl 2 / CH 3 OH=70 / 1) to obtain light yellow oil (898 mg), yield 51.05%. 1 H-NMR (400 MHz, CDCl 3 , ppm)δ: 0.67 (s, 3H), 0.86 (dd, 6H, J 1 = 6.8Hz, J 2 = 1.2 Hz), 0.91 (d, 3H, J = 6.4 Hz), 0.99 (s, 3H), 0.85-2.38 (remainingcholesterol protons), 2.75-2.79 (m, 4H), 2.91 -2.94 (m, 4H), 3.10-3.22 (m,2H), 3.47-3.83 (m, 85H), 4.25-4.27 (m, 4H), 5.34 (s, 1H).
Embodiment 3
[0036] Preparation of compound 5
[0037]
[0038] CH 2 Cl 2 (10 mL) solution, DCC (84 mg, 0.41 mmol), DMAP (8 mg, 0.065 mmol) were added and stirred for 30 minutes. Then, compound 4 (200 mg, 0.34 mmol) in CH was added dropwise to the mixture 2 Cl 2 (10 mL), stirred overnight at room temperature. After filtering off DCU, the reaction solution was concentrated. Finally, by silica gel column chromatography (CH 2 Cl 2 / CH 3 OH=100 / 1) to obtain light yellow oil (158 mg), yield 61.24%. 1H-NMR (400 MHz, CDCl 3 , ppm)δ:0.14-0.15 (m, 36H), 0.68 (s, 3H), 0.86 (d, 6H, J = 6.6 Hz), 0.91 (d, 3H, J =6.0 Hz), 0.99 (s, 3H), 0.85-2.39 (remaining cholesterol protons), 2.66-2.67(m, 4H), 2.75-2.79 (m, 4H), 2.91-2.94 (m, 4H), 3.10- 3.21 (m, 2H), 3.64-3.92(m, 95H), 4.03-4.07 (m, 2H) 4.25-4.27 (m, 6H), 4.35-4.38 (m, 1H), 4.42-4.50(m, 1H ), 5.00 (d, 1H, J =2.4 Hz), 5.34 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com