Butylphthalide Derivatives and Their Application in Preparation of Medicines for Protecting Nerve Cells
A kind of derivative, the technology of butylphthalide, applied in the application field of medicine, can solve the problems of elevated transaminase and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, the synthesis of derivative
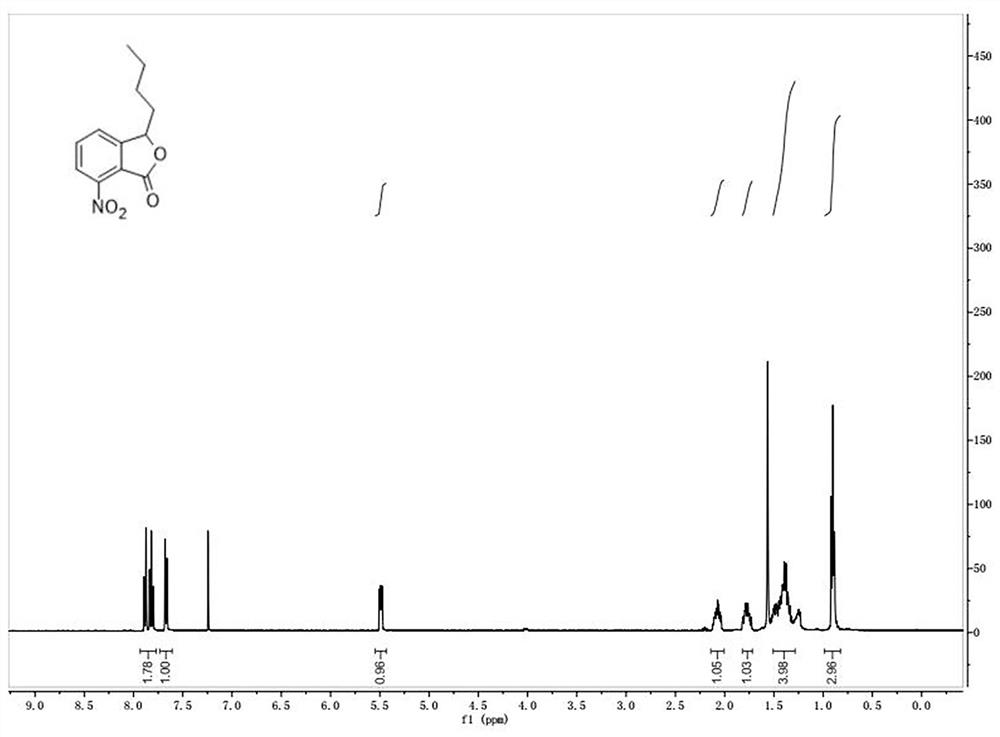
[0054] ①3-Butyl-7-nitroisobenzofuran
[0055] The chemical formula is as follows:
[0056]
[0057] The synthetic route is as follows:
[0058]
[0059] The picture of the quality inspection result is as follows figure 1 shown.
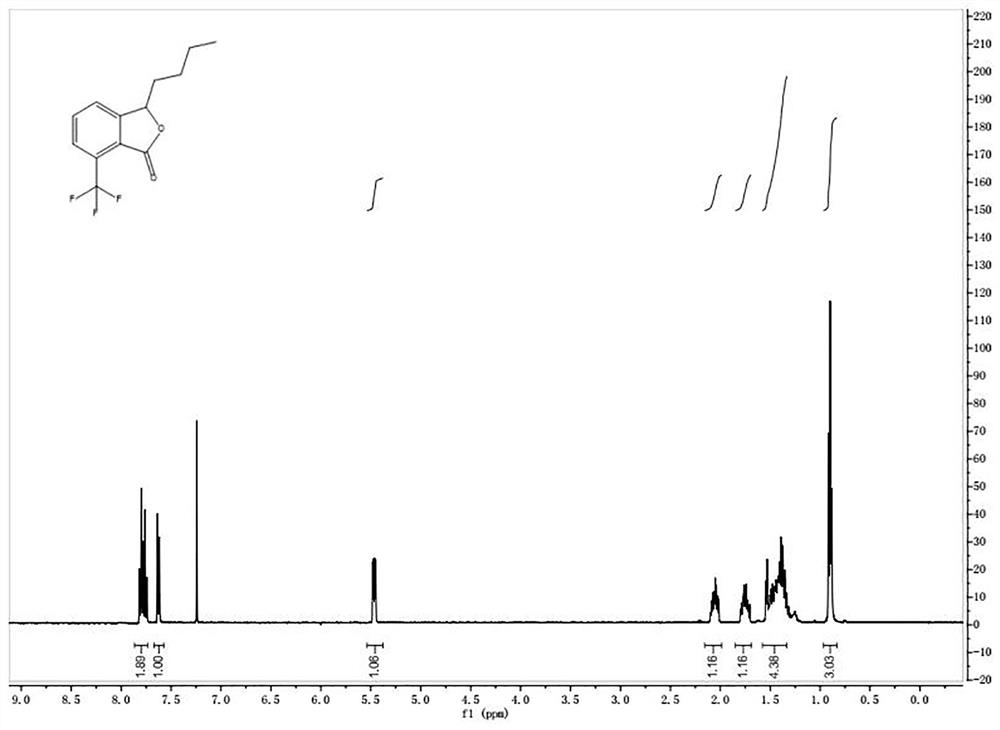
[0060] ②3-Butyl-7-(trifluoromethyl)isobenzofuran
[0061] The chemical formula is as follows:
[0062]
[0063] The synthetic route is as follows:
[0064]
[0065] The picture of the quality inspection result is as follows figure 2 shown.
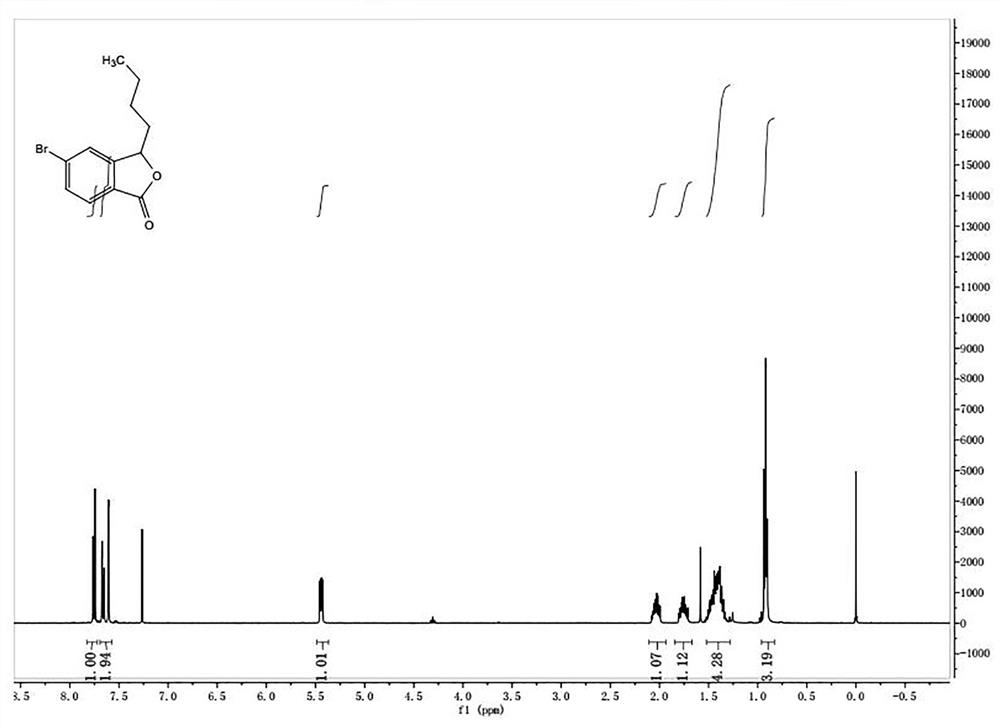
[0066] ③3-Butyl-5-bromoisobenzofuranone
[0067] The chemical formula is as follows:
[0068]
[0069] The synthetic route is as follows:
[0070]
[0071] The picture of the quality inspection result is as follows image 3 shown.
[0072] ④3-Butyl-5-(trifluoromethyl)isobenzofuranone
[0073] The chemical formula is as follows:
[0074]
[0075] The synthetic route is as follows:
[0076]
[0077] The picture of the qualit...
Embodiment 2
[0079] Example 2. Detection test of butylphthalide derivatives on Aβ-induced anti-apoptotic MTT activity of SY5Y nerve cells
[0080] Experimental steps:
[0081] 1. SHY5Y cells were treated with 1*10 5 piece / cm 2 Density inoculated in 96-well plate and cultivated for 24h.
[0082] 2. When the confluence of the cells reaches 80%~90%, discard the medium, wash with PBS 3 times, add drugs (0.5mM, diluted in normal medium DMEM), and incubate at 37°C for 4h.
[0083] 3. Discard the drug, wash twice with PBS, and add 20 μM Aβ 25-35 (Add PBS in advance to dissolve, incubate at 37°C for 2 days) and incubate with the drug mixture at 37°C for 48h, in which Aβ 25-35 group as a model control. In addition, a PBS group (edge holes were filled with sterile PBS to eliminate edge effects) and a blank control group (normal cells were cultured in normal medium without any treatment) were set up.
[0084] 4. Add 10 μL of MTT solution (5 mg / mL) prepared in PBS to each well, and incubate ...
Embodiment 3
[0089] Example 3, butylphthalide derivatives release test of active oxygen on Aβ-induced SY5Y nerve cell damage
[0090] According to the results of the MTT experiment, the nerve cell injury reactive oxygen species release experiment was further tested. In this experiment, all compounds were detected at the same time, but the cells in group ③ were not involved in the analysis due to more cell death.
[0091] Experimental steps:
[0092] 1. SHY5Y cells were treated with 1*10 5 piece / cm 2 Density inoculated in 96-well plate and cultivated for 24h.
[0093] 2. When the confluence of the cells reaches 80%~90%, discard the medium, wash with PBS 3 times, add drugs (0.5mM, diluted in normal medium DMEM) and incubate at 37°C for 4h.
[0094] 3. Discard the drug, wash twice with PBS, and add 20 μM Aβ 25-35 (Add PBS in advance to dissolve, incubate at 37°C for 2 days) and incubate with the drug mixture at 37°C for 48h, in which Aβ 25-35 group as a model control. In addition, a P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com