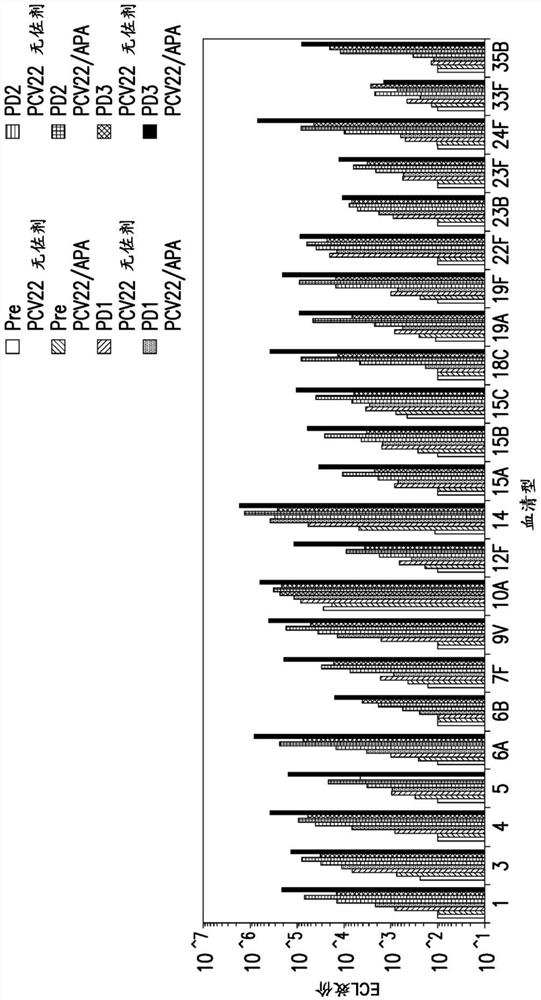
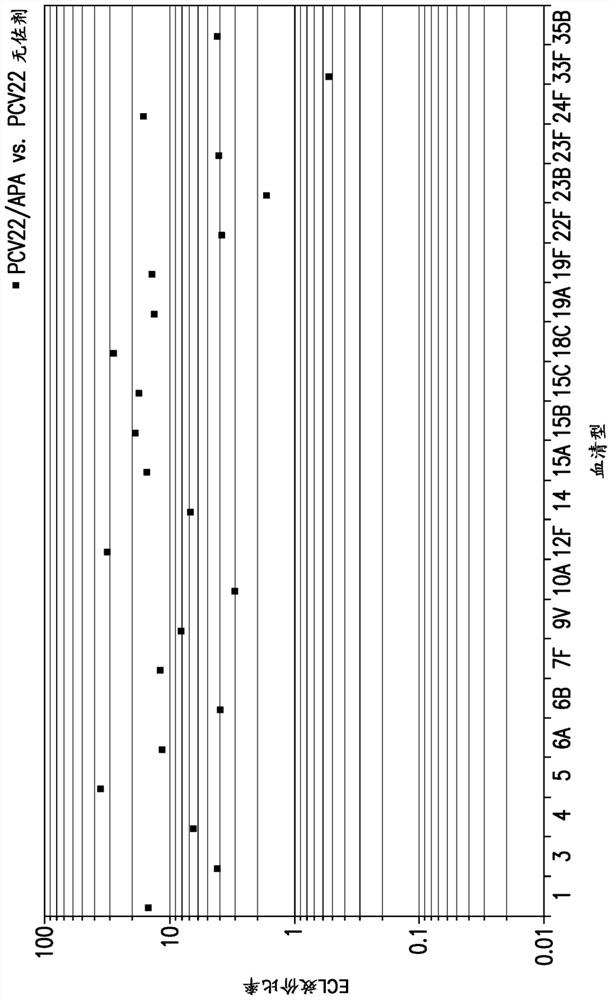
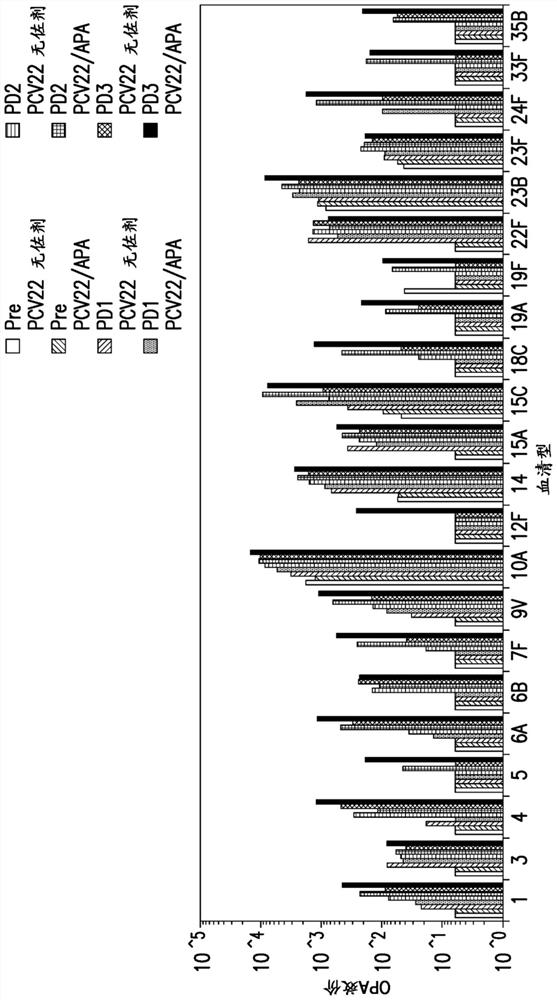
Compositions comprising streptococcus pneumoniae polysaccharide-protein conjugates and methods of use thereof
一种肺炎链球菌、多糖蛋白的技术,应用在药物组合、含有效成分的医用配制品、非有效成分的医用配制品等方向,能够解决肺炎球菌流行率增加等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0438] Preparation of Streptococcus pneumoniae capsular polysaccharide
[0439] Methods of culturing pneumococci are well known in the art. See, eg, Chase, 1967, Methods of Immunology and Immunochemistry 1:52. Methods of preparing pneumococcal capsular polysaccharides are also well known in the art. See, eg, European Patent No. EP 0 497 524 B1. The method described below generally follows that described in European Patent No. EP0 497524 B1 and is generally applicable to all pneumococcal serotypes.
[0440] Isolation of pneumococcal strains of serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15A, 15B, 18C, 19A, 19F, 22F, 23F, 33F, and 35B Strains were obtained from the Merck Culture Collection. Serotype 23B strains were obtained from the Centers for Disease Control and Prevention and the University of Alabama at Birmingham. Serotype 24F strains were obtained from the Merck Culture Collection and the University of Alabama at Birmingham. Subtypes can be different...
Embodiment 2
[0444] Purification of pneumococcal polysaccharide
[0445] Purification of pneumococcal polysaccharides consists of multiple centrifugation, depth filtration, concentration / diafiltration operations and precipitation steps. All steps were performed at room temperature unless otherwise stated.
[0446] Use cationic polymers (such as BPA-1000, (Baker Hughes Inc., Houston, TX), Spectrum 8160, poly(ethyleneimine), and Millipore pDADMAC) were flocculated of inactivated media from fermentor cultures of S. pneumoniae. Cationic polymers bind to impurity proteins, nucleic acids and cellular debris. After the flocculation step and aging phase, the flocculated solids are removed by centrifugation and multiple depth filtration steps. Clarified medium was concentrated and diafiltered using 100 kDa to 500 kDa MWCO (molecular weight cut off) filters. Diafiltration is using Tris, MgCl 2 buffer and sodium phosphate buffer. Diafiltration removes residual nucleic acids and proteins.
[0...
Embodiment 3
[0449] Preparation of serotype 1 conjugates for polyvalent studies of PCV23 (DMSO) using DMSO conjugation
[0450] Polysaccharides are solubilized, size reduced to target molecular weight, chemically activated and buffer exchanged by ultrafiltration. Activated polysaccharide and purified CRM197 were separately lyophilized and then redissolved in dimethyl sulfoxide (DMSO). The redissolved polysaccharide and CRM197 solutions were then combined and conjugated as described below. The resulting conjugate was purified by ultrafiltration before a final 0.2 micron filtration. Multiple process parameters such as pH, temperature, concentration and time are controlled in each step to produce conjugates with desired properties.
[0451] Polysaccharide size reduction and oxidation
[0452] The purified pneumococcal capsular Ps powder was dissolved in water and subjected to 0.45 micron filtration. The dissolved polysaccharides were homogenized to reduce the Ps molecular weight. The hom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com