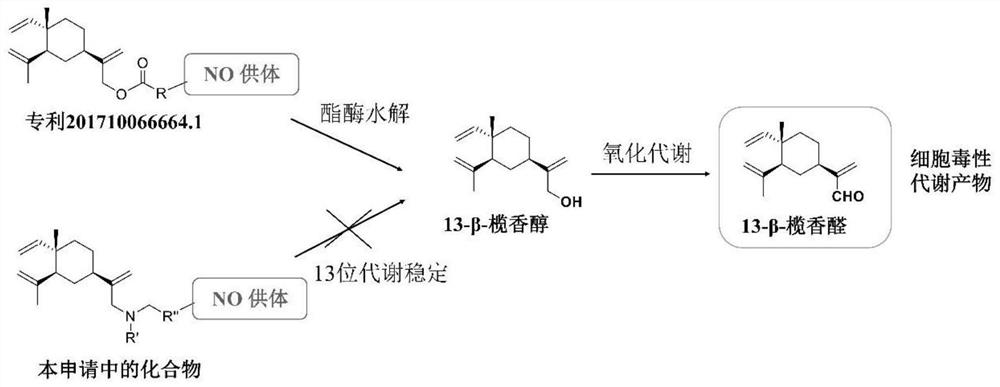
14-chloro-beta-elemene nitric oxide donor type derivative as well as preparation and application thereof
A nitric oxide and elemene technology is applied in organic chemistry, drug combination, medical preparations containing active ingredients, etc. It can solve the problems of poor stability of compounds, death of animals administered with drugs, and high safety risks, etc., and achieve good results. Therapeutic activity, improving in vivo stability, and improving the effect of antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
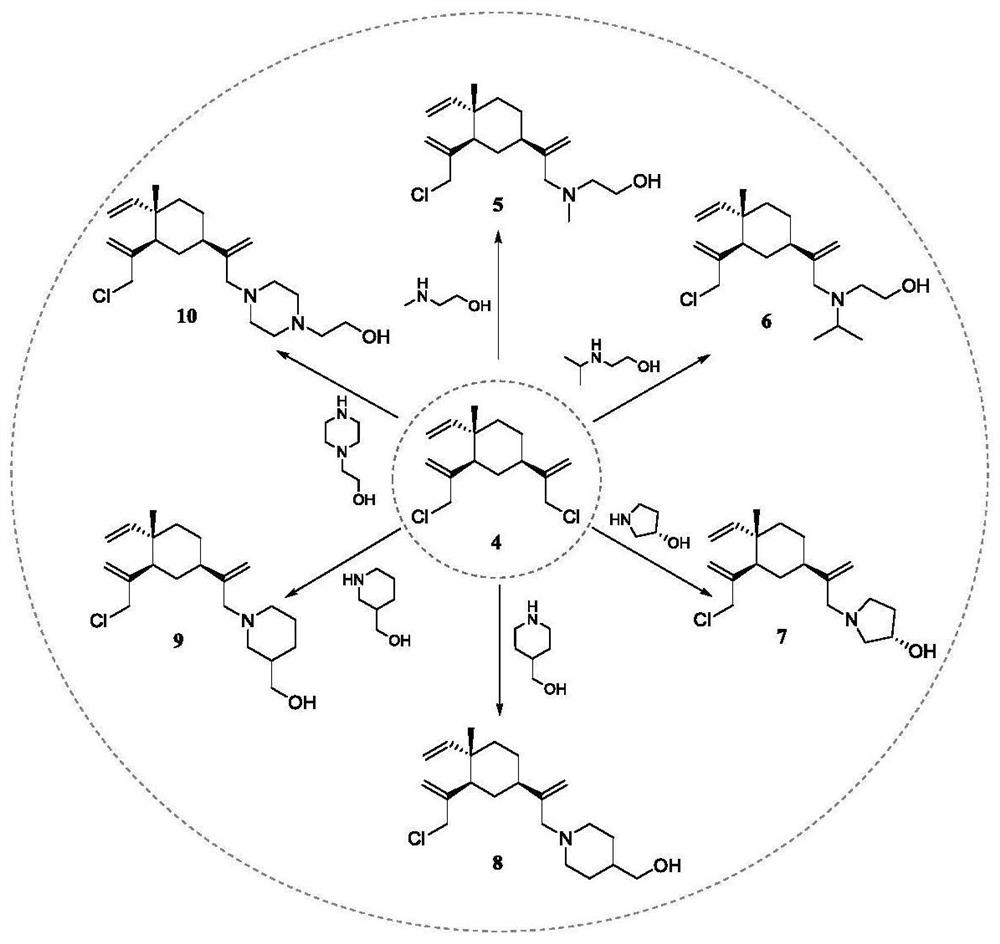
[0042] (1) Preparation of Intermediate 4
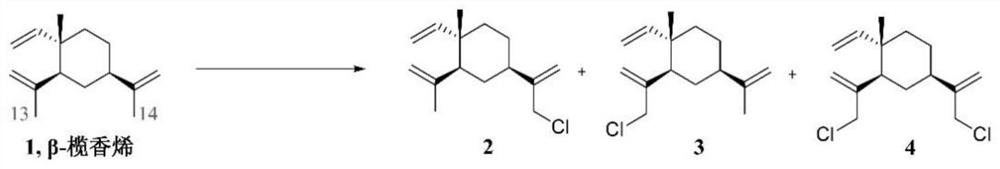
[0043] like figure 2 As shown, at 0 °C, to a mixed solution of β-elemene (1.02 g, 5 mmol) in dichloromethane (8 mL) and tetrahydrofuran (2 mL), were added NCS (1.34 g, 20 mmol), trifluoro Ytterbium methanesulfonate (310 mg, 0.5 mmol) and trimethylchlorosilane (54 mg, 0.5 mmol) were reacted at 0° C. for 8 h. At the end of the reaction, the solvent was distilled off under reduced pressure, diluted with water (15 mL), extracted with ethyl acetate (4 mL×3), the organic phases were combined, washed with water (20 mL×2) and saturated brine (20 mL×2) in turn, After drying over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and the residue was separated by silica gel column chromatography (pure petroleum ether) to obtain a colorless liquid compound, namely Intermediate 4, with a yield of 45%.
[0044] 1 H NMR (400MHz, CDCl 3 )δ5.85–5.72(m,1H),5.28(s,1H),5.18(s,1H),5.04(s,1H),4.98–4.89(m,3H),4.15–4.05(m,3H...
Embodiment 1
[0083]
[0084] 4-(2-((4-(2-((2-((1R,3R,4S)-3-(3-chloroprop-1-en-2-yl)-4-methyl-4-ethene cyclohexyl)))(methyl)amino)ethoxy)-4-oxobutyryl)oxy)ethoxy)-3-(benzenesulfonyl)-1,2,5-oxadiazole 2- Preparation of oxides.
[0085] To a solution of intermediate 5 (26 mg, 0.084 mmol) in dichloromethane (1.5 mL), intermediate 14a (39 mg, 0.101 mmol), DMAP (1 mg, 0.008 mmol), EDCI (24 mg, 0.126 mmol) were added sequentially, Stir at room temperature for 8h. Dichloromethane (5 mL) was added to dilute, washed with water (10 mL×2) and saturated brine (10 mL×2) in turn, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was separated by silica gel column chromatography (dichloromethane). Methane:methanol volume ratio 400:1), pale yellow liquid, yield 69%.
[0086] 1 H NMR (400MHz, CDCl 3 )δ8.07(d,J=7.8Hz,2H),7.75(d,J=7.5Hz,1H),7.63(t,J=7.8Hz,2H),5.78(dd,J=17.8,10.4Hz, 1H), 5.26 (s, 1H), 5.02–4.87 (m, 5H), 4.65–4.59 (m, 2H), 4.57–4...
Embodiment 2
[0088]
[0089] 4-(2-((5-(2-((2-((1R,3R,4S)-3-(3-chloroprop-1-en-2-yl)-4-methyl-4-ethene cyclohexyl)allyl)(methyl)amino)ethoxy)-5-oxopentanoyl)oxy)ethoxy)-3-(benzenesulfonyl)-1,2,5-oxadi The preparation method of oxazole 2-oxide is basically the same as that in Example 1, the difference is that the succinic anhydride in (3) is replaced with glutaric anhydride.
[0090] In this example, a yellow waxy liquid was prepared with a yield of 71%.
[0091] 1 H NMR (400MHz, CDCl 3 )δ8.06(d,J=8.5Hz,2H),7.76(t,J=7.5Hz,1H),7.63(t,J=7.9Hz,2H),5.87–5.71(m,1H),5.26( s, 1H), 5.01–4.86 (m, 5H), 4.67–4.60 (m, 2H), 4.53–4.46 (m, 2H), 4.17 (t, J=5.7Hz, 2H), 4.09 (d, J= 11.6Hz, 1H), 3.96 (d, J=11.7Hz, 1H), 2.95 (s, 2H), 2.57 (t, J=5.1Hz, 2H), 2.43 (dt, J=17.0, 7.3Hz, 4H) ,2.28(dd,J=11.9,4.0Hz,1H),2.16-2.07(m,1H),2.02-1.93(m,2H),1.65-1.35(m,6H),0.96(s,3H). 13 CNMR (100MHz, CDCl 3 )δ173.0,172.8,158.8,151.4,149.4,147.8,138.1,135.8,129.8,128.7,116.3,110.9,110.5,69.0,63.3,62.5,61.2,55.4,51.1,...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap