Gene therapy for alzheimer's disease
A technology of gene and composition, applied in the field of composition for the treatment of Alzheimer's disease and other neurodegenerative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
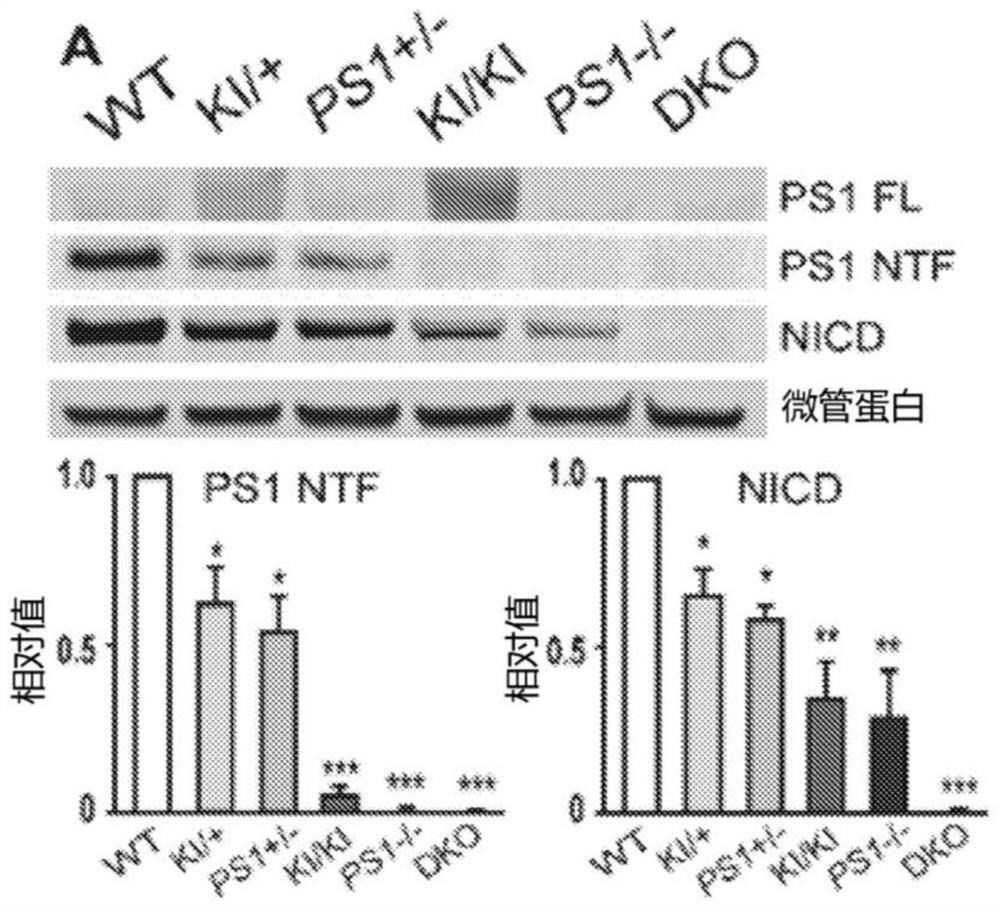
[0125] Example 1: Dose-dependent rescue of γ-secretase activity in MEFs with various PS genotypes: PS1 + / + , PS1 L435F / + , PS1 + / - , PS1 L435F / L435F , PS1 - / - and PS1 - / - ; PS2 - / -
[0126] To determine whether the reduced γ-secretase activity associated with PSEN1 mutations could be corrected by introducing wild-type (WT) hPS1, primary MEFs from embryos carrying various PS genotypes were derived: PS1 + / + , PS1 L435F / + , PS1 + / - , PS1 L435F / L435F , PS1 - / - and PS1 - / - ; PS2 - / - (DKO). Immortalized MEFs were transiently transfected with CMV-NΔE, and γ-secretase activity was assessed by measuring the levels of NICD and PS1 NTF / CTF. NICD levels decreased in a PS1 dose-sensitive manner and were undetectable in DKO cells ( Figure 1A ). NICD level at PS1 L435F / L435F MEF (“L435F KI / KI” MEF) and PS1 - / - Decreased but detectable in MEF ( Figure 1A ), however by using the L435F KI / KI and PS1 - / - In vitro γ-secretase assays of embryonic brains failed to detect de novo...
Embodiment 2
[0128] Example 2: Development of an optimized wild-type human PS1 expression system in vitro
[0129] method
[0130] Cell Culture and Transfection
[0131] Psen-null mouse embryonic fibroblasts (MEFs) lacking endogenous PS1 and PS2 were maintained in DMEM supplemented with 10% FBS and were cultured with or without γ-secretase reporter using Lipofectamine 3000 according to the instructions. In the case of the gene CMV-NΔE, plasmids expressing wild-type endogenous hPSEN1 cDNA (wt_PS1) or codon-optimized hPSEN1 cDNA (opti_PS1) were transiently transfected. Cell lysates were collected at the 24 hour time point.
[0132] western blot
[0133] Cell lysates were subjected to SDS-PAGE and proteins were transferred to nitrocellulose membranes. After blocking in TBST / 5% non-fat dry milk, membranes were incubated with primary antibodies overnight. To control loading, membranes were stripped and re-probed with an anti-β-actin antibody. Band intensities were quantified using ImageJ ...
Embodiment 3
[0138] Example 3: Development of optimized wild-type human PS1 expression system in vivo
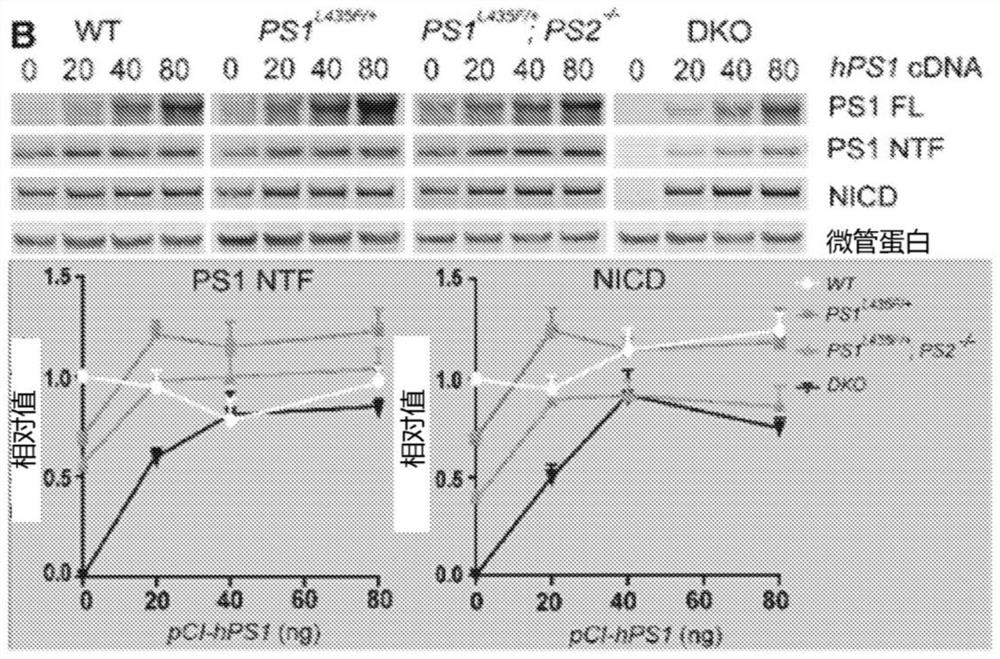
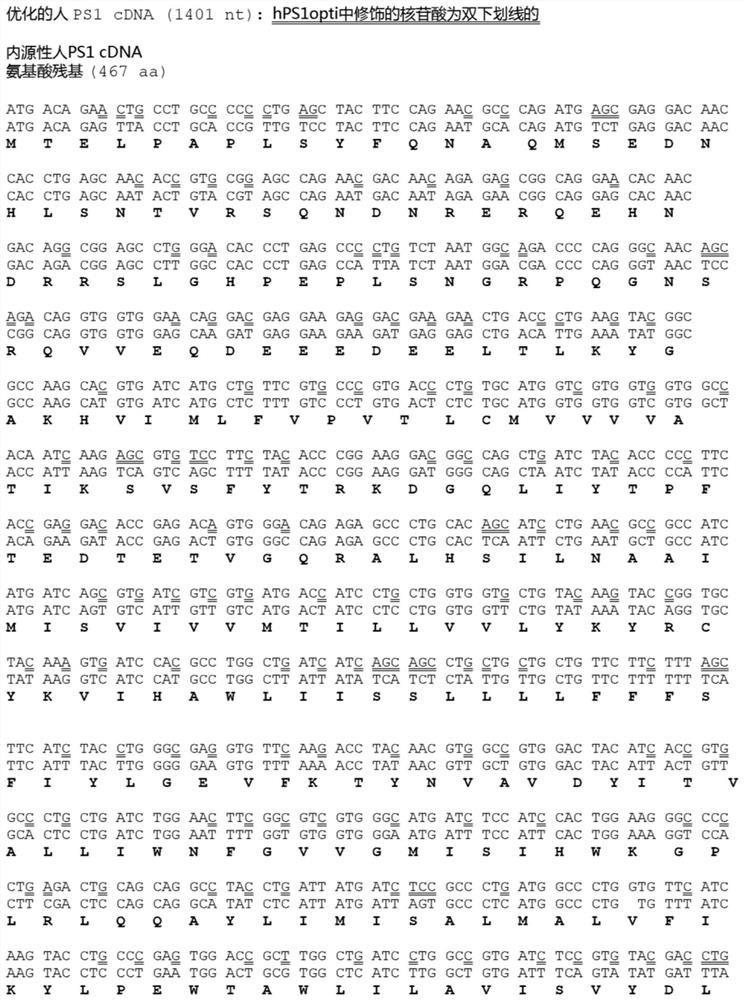
[0139] Transgenic mice were developed that constitutively or inducibly express human PSEN1 wild-type cDNA under the control of the CAMK2A promoter. To maximize PS1 production and activity, hPS1 was codon-optimized (hPS1opti) and then compared to endogenous hPS1 by co-transfecting increasing amounts of pCI-hPS1 or pCI-hPS1opti and CMV-NΔE into PS DKO MEFs PS1 levels and γ-secretase activity between hPS1opti cDNA and hPS1opti cDNA. Relative to endogenous hPS1 cDNA, hPS1 opti cDNA elicited higher levels of PS1 NTF and higher γ-secretase activity, as measured by NICD production (Figure 3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com