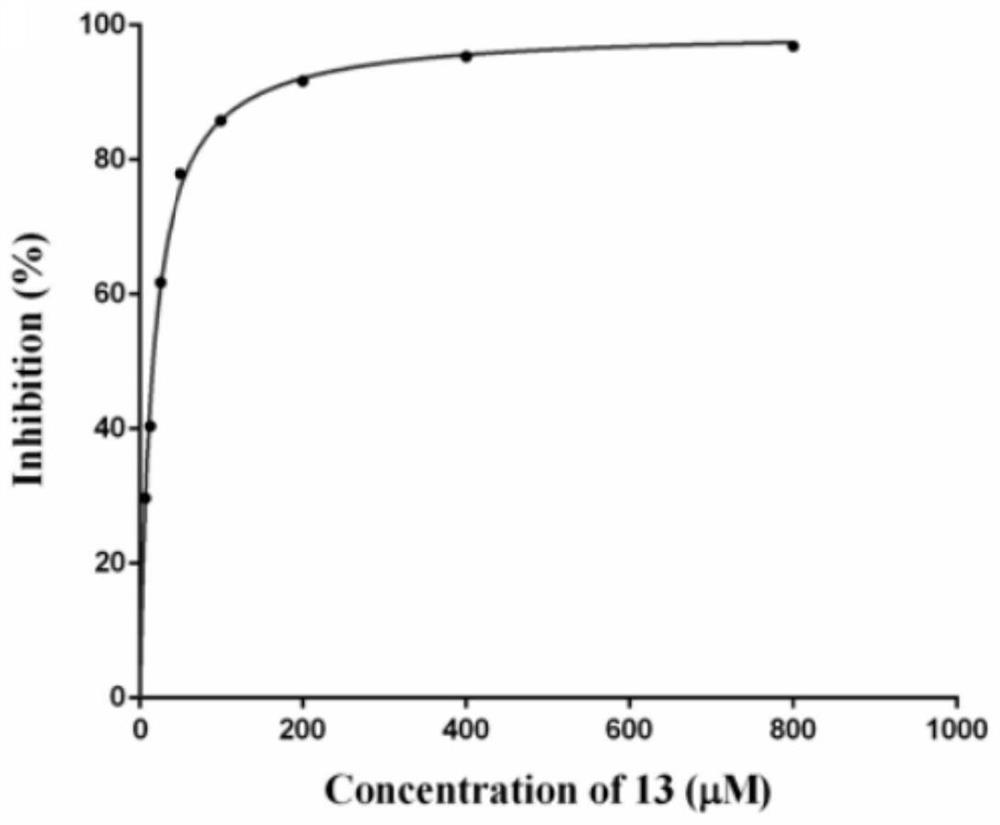
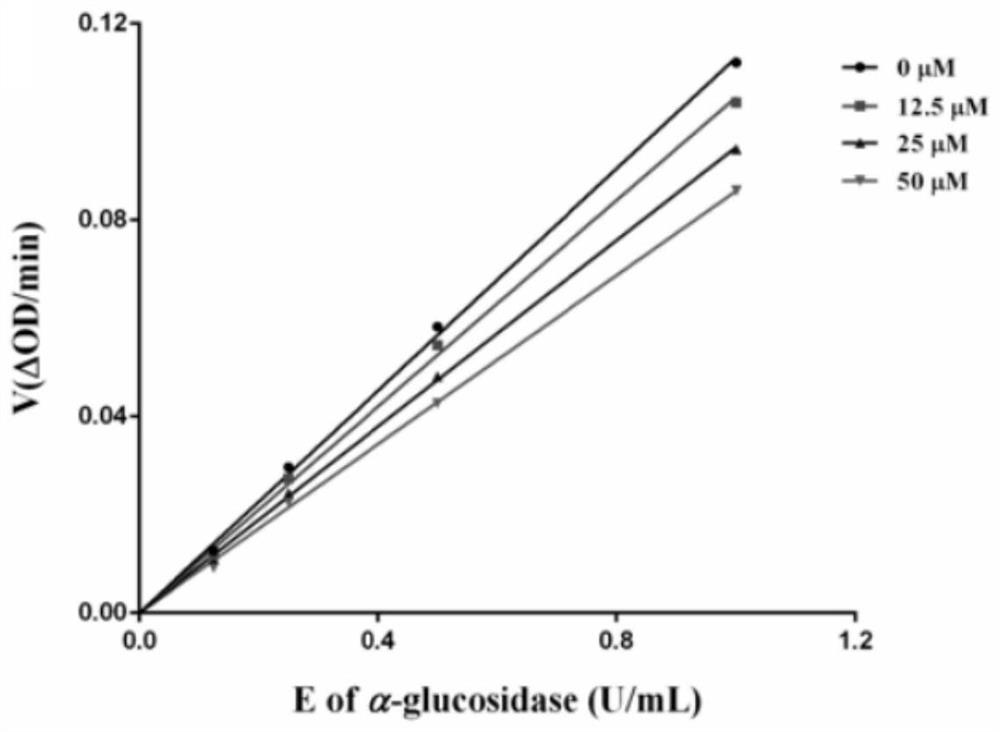
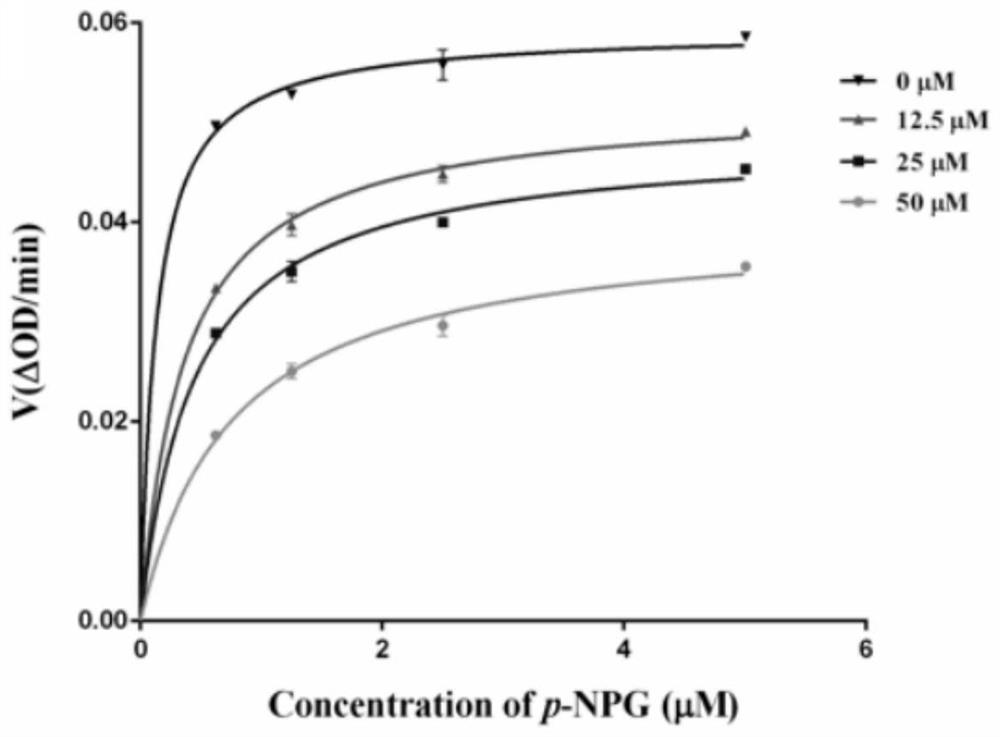
Novel glycoside compound, pharmaceutical composition, preparation method and application
A technology of glycosides and compounds, applied in the field of medicine, to achieve obvious inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1. The preparation method of glycoside compound Euphorbiacetophenone E and 1,2,3-tri-O-galloyl-β-D-glucopyranose
[0056] Step S1: take the root of Euphorbia chamaejasma, dry and pulverize, add 8-15 times the amount of solvent to reflux for extraction for 1-3 hours, repeat 2-4 times, combine the extracts, filter, and concentrate under reduced pressure to obtain a concentrate; extract The temperature is 65°C-85°C;
[0057] The extraction solvent can be selected from water, methanol, ethanol or mixtures thereof, preferably 60-95% ethanol; (10 times the amount of 80% ethanol solvent is added in this embodiment, reflux extraction for 2 hours, repeated 3 times).
[0058] Step S2: Extract the concentrated solution with an organic solvent for 2-5 times, combine the organic phases, recover the organic solvent, and obtain the extract III; the extraction temperature is from 65°C to the boiling point of the extraction solvent;
[0059] The organic extraction solvent ca...
Embodiment 2
[0065] Others are the same as Example 1, the difference is:
[0066] Step S1: In this embodiment, 8 times the amount of 50% ethanol solvent was added, and the extraction was carried out under reflux for 1 hour, and repeated twice.
[0067] Step S2: In this example, the concentrated solution was extracted twice with an organic solvent, and the solvent was propyl acetate.
[0068] Step S3: In this embodiment, the eluent of silica gel column chromatography is petroleum ether-acetone, the eluent of reverse phase silica gel column chromatography is methanol-water, and the material of Sephadex Gel column chromatography is Sephadex G -10, the eluent is dichloromethane-methanol.
[0069] Step S4: In this embodiment, a C4 liquid chromatography column is selected, and acetonitrile-water is selected as the mobile phase.
Embodiment 3
[0071] Others are the same as Example 1, the difference is:
[0072] Step S1: In this example, 15 times the amount of 95% ethanol solvent was added, and the reflux extraction was performed for 3 hours, which was repeated 4 times.
[0073] Step S2: In this example, the concentrated solution was extracted 5 times with an organic solvent, and the solvent was propyl acetate.
[0074] Step S3: In this embodiment, the eluent of silica gel column chromatography is cyclohexane-ethyl acetate, the eluent of reverse phase silica gel column chromatography is methanol-water, and the material of Sephadex column chromatography It is Sephadex G-10, and the eluent is petroleum ether-chloroform-methanol.
[0075] Step S4: In this embodiment, a C6 liquid chromatography column is selected, and acetonitrile-water is selected as the mobile phase.
[0076] It should be noted that Examples 2-3 of the present invention and other preparation method examples not shown in the present invention are all ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com