Cyclic mRNA (messenger ribonucleic acid) tumor immune drug for colorectal cancer
A colorectal cancer and tumor technology, applied in the field of potential colorectal cancer tumor immune drugs, to achieve the effect of reducing social security pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
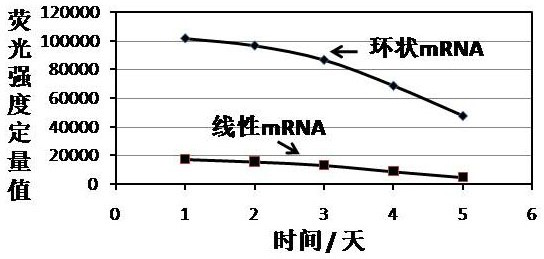
[0116] Example 1 Expression of circular mRNA mediated by IRES in 293T cells
[0117] The first step is to construct a regulatory sequence (SEQ ID 52), a 5´ homology arm (SEQ ID 53), a 3´ intron (SEQID 54), a second exon (SEQ ID 55), and a 5´ spacer (SEQ ID 56), the IRES element with the nucleotide sequence shown in SEQ ID 51, EGFP coding region (SEQ ID 57), 3' spacer (SEQ ID 58), the first exon (SEQ ID59), 5 ´ intron (SEQ ID 60), 3´ homology arm (SEQ ID 61), and the restriction site XbaI (SEQ ID 62) that can be used for plasmid linearization to express the target gene of the antigen polypeptide, and connect the resulting gene fragments to the pUC57 vector;
[0118] In the second step, after activating the synthetic puncture bacteria at 36.8 degrees Celsius and 200 rpm for 4 hours, take the activated bacteria liquid and cultivate it overnight at 36.8 degrees Celsius and 200 rpm. Then, the plasmid was extracted, and the OD value and plasmid extraction concentration (342.1 ng p...
Embodiment 2
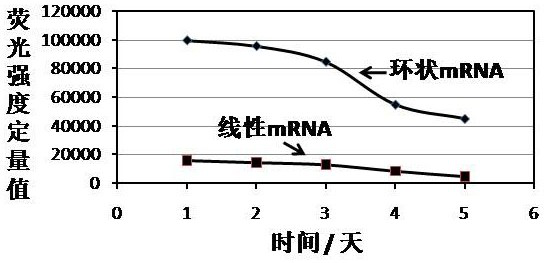
[0126] Example 2 Expression of circular mRNA mediated by IRES in 293T cells
[0127] The first step is to construct a regulatory sequence (SEQ ID 52), a 5´ homology arm (SEQ ID 53), a 3´ intron (SEQID 63), a second exon (SEQ ID 55), and a 5´ spacer (SEQ ID 56), the IRES element with the nucleotide sequence shown in SEQ ID 51, EGFP coding region (SEQ ID 57), 3' spacer (SEQ ID 58), the first exon (SEQ ID59), 5 ´ intron (SEQ ID 60), 3´ homology arm (SEQ ID 61), and the restriction site XbaI (SEQ ID 62) that can be used for plasmid linearization to express the target gene of the antigen polypeptide, and connect the resulting gene fragments to the pUC57 vector;
[0128] In the second step, after activating the synthetic puncture bacteria at 36.8 degrees Celsius and 200 rpm for 4 hours, take the activated bacteria liquid and cultivate it overnight at 36.8 degrees Celsius and 200 rpm. Then, the plasmid was extracted, and the OD value and plasmid extraction concentration (282.1 ng p...
Embodiment 3
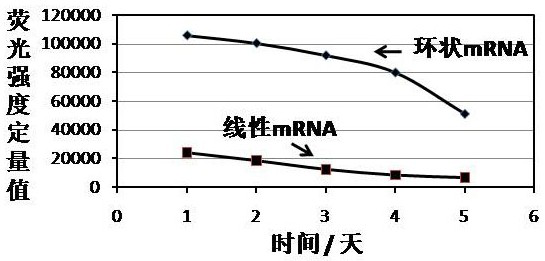
[0136] Example 3 Expression of circular mRNA in 293T cells mediated by IRES
[0137] The first step is to construct a regulatory sequence (SEQ ID 52), a 5´ homology arm (SEQ ID 53), a 3´ intron (SEQ ID 63), a second exon (SEQ ID 64), and a 5´ spacer (SEQ ID 56), the IRES element with the nucleotide sequence shown in SEQ ID 51, EGFP coding region (SEQ ID 57), 3' spacer (SEQ ID 58), the first exon (SEQ ID59), 5 ´ intron (SEQ ID 60), 3´ homology arm (SEQ ID 61), and the restriction site XbaI (SEQ ID 62) that can be used for plasmid linearization to express the target gene of the antigen polypeptide, and connect the resulting gene fragments to the pUC57 vector;
[0138] In the second step, after activating the synthetic puncture bacteria at 36.8 degrees Celsius and 200 rpm for 4 hours, take the activated bacteria liquid and cultivate it overnight at 36.8 degrees Celsius and 200 rpm. Then, the plasmid was extracted, and the OD value and plasmid extraction concentration (302.4 ng ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com