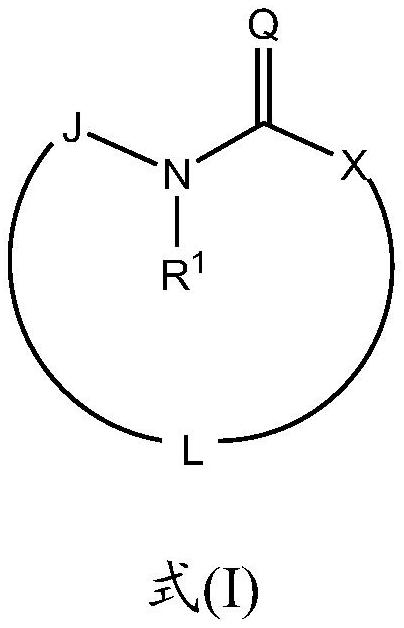
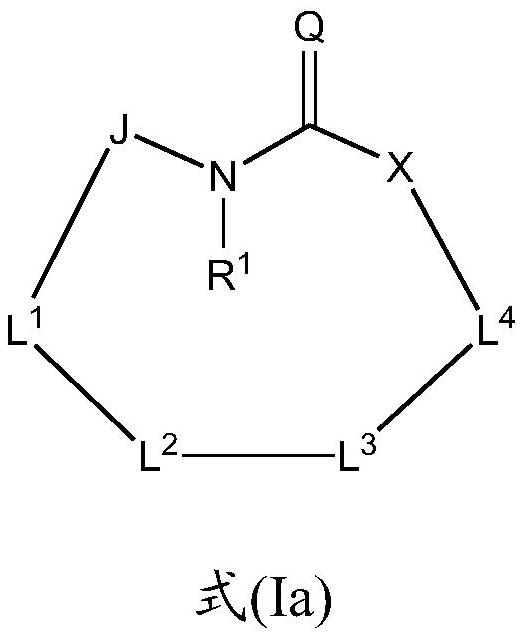
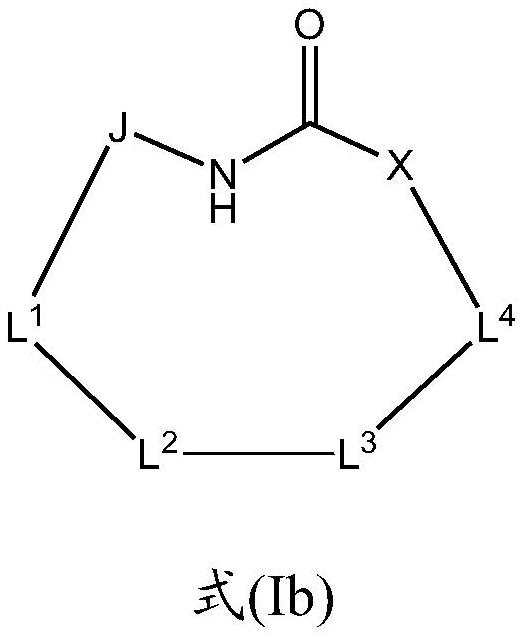
Macrocyclic sulfonamide derivatives useful as NLRP3 inhibitors
A monocyclic, compound technology for the treatment and prevention of medical conditions and diseases, the field of macrocyclic sulfonamides, which can solve problems such as limited efficacy and non-specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[2056] Example 1: 16,16-Dimethyl-6-(propan-2-yl)-18-oxa-11λ 6 -Thia-10,15,20,24-tetraaza-tetracyclo[17.3.1.1 12,15 .0 2,7 ]Tetracos-1(22),2(7),3,5,12(24),13,19(23),20-octene-9,11,11-trione
[2057]
[2058] DMAP (70.8 mg, 0.580 mmol) and EDC (111 mg, 0.580 mmol) were added to 2-(2-isopropyl-6-(2-(2-methyl-2-(3-sulfamoyl-1H- Pyrazol-1-yl)propoxy)pyridin-4-yl)phenyl)acetic acid (Intermediate C2) (137 mg, 0.290 mmol) in DMF (2 mL), the reaction was stirred at room temperature for 18 h. The crude product was purified by acidic preparative HPLC (50-80% MeCN in water) to give the title compound (10.78 mg, 8%) as a flocculent white solid.
[2059] LCMS m / z 455.2(M+H) + (ES + ); 453.2 (M-H) - (ES - ).
[2060] 1 H NMR (DMSO-d 6 )δ11.93(s, 1H), 8.22(d, J=5.1Hz, 1H), 8.03(s, 1H), 7.38-7.32(m, 1H), 7.29(t, J=7.6Hz, 1H), 6.94-6.90(m, 1H), 6.88(d, J=5.1Hz, 1H), 6.71(s, 1H), 5.73(s, 1H), 4.50(s, 2H), 3.41-3.35(m, 2H) , 2.97-2.92 (m, 1H), 1.66 (s, 6H), 1.17 (d, J=6.7Hz, 6H)....
Embodiment 6
[2069] Example 6: 16-Fluoro-19,19-dimethyl-21-oxa-14λ 6 -Thia-13,18,23,27-tetraazapentacyclo[20.3.1.1 15,18 .0 2,10 .0 5,9 ] Heptadecan-1(25),2,4,9,15(27),16,22(26),23-octene-12,14,14-trione
[2070]
[2071]2-(5-(2-(2-(4-Fluoro-3-sulfamoyl-1H-pyrazol-1-yl)-2-methylpropoxy)pyridin-4-yl)-2, 3-Dihydro-1H-inden-4-yl)acetic acid (Intermediate C6) (45 mg, 0.092 mmol) was dissolved in NMP (2 mL), to which was added HATU (52 mg, 0.137 mmol) and DIPEA (48 μL, 0.275 mmol) ). The reaction was stirred at room temperature for 18 h. The mixture was purified by basic preparative HPLC (20-50% MeOH in water) to give the title compound (8 mg, 18%) as a white solid.
[2072] LCMS m / z 471.4(M+H) + (ES + ).
[2073] 1 H NMR (DMSO-d 6 )δ12.12(s, 1H), 8.28(d, J=4.2Hz, 1H), 8.20(d, J=5.1Hz, 1H), 7.19(d, J=7.5Hz, 1H), 6.90(d, J=7.5Hz, 1H), 6.86(dd, J=5.2, 1.4Hz, 1H), 5.85(s, 1H), 4.53(s, 2H), 3.38(s, 2H), 2.93(t, J=7.4 Hz, 2H), 2.80 (t, J=7.5Hz, 2H), 2.03 (p, J=7.5Hz, 2H), 1.63 (s, ...
Embodiment 17
[2074] Example 17: 17-(2-Hydroxypropan-2-yl)-22-oxa-14λ 6 -Thia-13,24-diazapentacyclo-[21.3.1.1 15,19 .0 2,10 .0 5,9 ] Octacos-1(26),2,4,9,15,17,19(28),23(27),24-nonene-12,14,14-trione
[2075]
[2076] CDI (57 mg, 0.352 mmol) was dissolved in MeCN (10 mL), to which was added 2-(5-(2-(3-(2-hydroxypropan-2-yl)-5-sulfamoylphenethoxy) )pyridin-4-yl)-2,3-dihydro-1H-inden-4-yl)acetic acid (Intermediate C17) (192 mg, 0.377 mmol). The mixture was stirred at room temperature for 1 h. A solution of DBU (53 μL, 0.355 mmol) in MeCN (10 mL) was then added and the mixture was stirred at room temperature for 24 h. The reaction was concentrated in vacuo and the resulting residue was purified by acidic preparative HPLC (35-65% MeCN in water) to give the title compound (6 mg, 3%) as a white solid.
[2077] LCMS m / z 493.4(M+H) + (ES + ).
[2078] 1 H NMR (CDCl 3 )δ7.97-7.87(m, 2H), 7.85-7.73(m, 2H), 7.47(s, 1H), 7.29(s, 1H), 7.06(d, J=7.8Hz, 1H), 6.69(d , J=5.2Hz, 1H), 6.46(br ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap