Method for simultaneously realizing gene editing and transcriptional regulation by using I-type CRISPR-Cas system
A technology of transcriptional regulation and gene editing, applied in the biological field, can solve problems such as inability to complete gene editing and transcriptional regulation at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The crtB gene of S. spainata, as shown in SEQ ID NO: 1 of the sequence listing, encodes a phytoene synthase (HAH_2563). Knockout of this gene will block the expression of carotenoids and turn the cells from red to white.
[0075] Both the Cascade effector and the Cas3 endonuclease are endogenous to H. spainii.
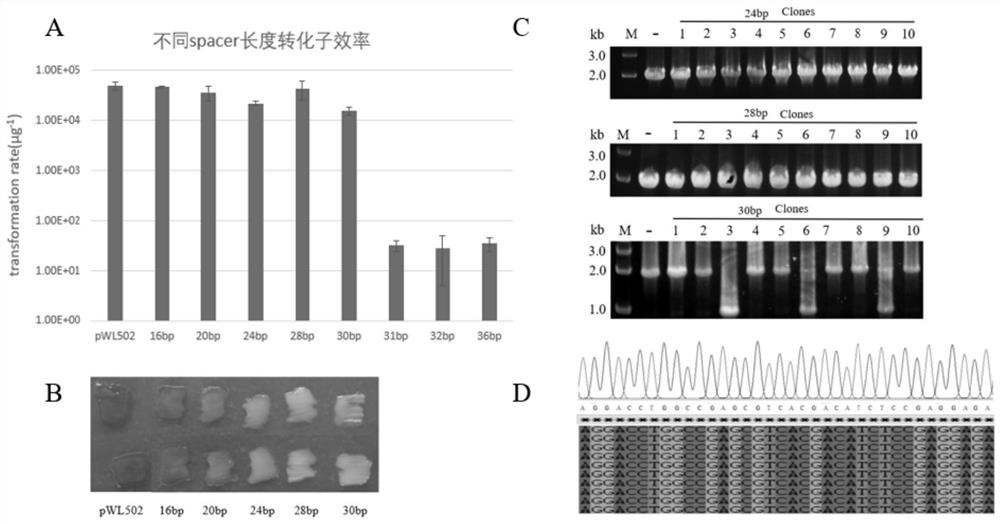
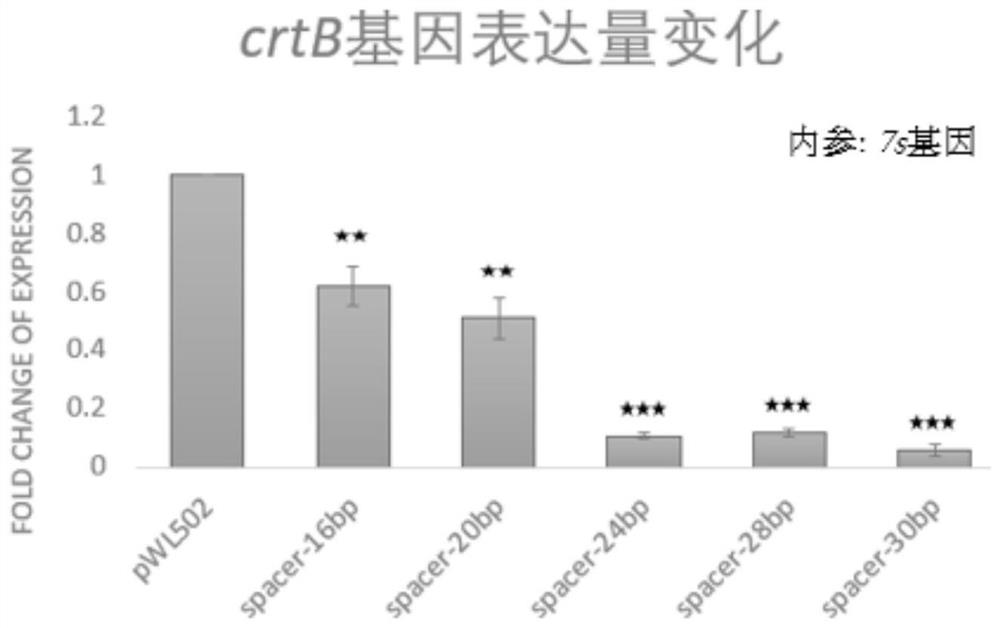
[0076]In this example, for the crtB gene of S. spain, using its own type I-B CRISPR-Cas system, by using gRNAs with spacers of different lengths, different degrees of transcriptional inhibition of the crtB gene can be achieved without cleaving it.
[0077] 1. Construction of plasmids for expressing gRNAs with spacers of different lengths
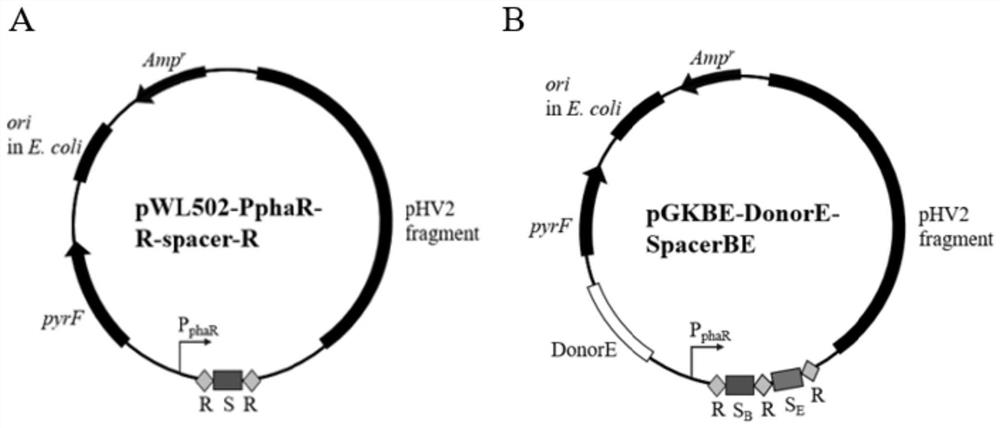
[0078] 1. Preparation of seven PphaR-R-spacer-R fragments
[0079] Our laboratory has constructed a gRNA expression plasmid pWL502-PphaR-R-C1-R (namely plasmid pGK) targeting the crtB gene of the DLCR strain of S. spainata in the early stage, which contains the PphaR-R-C1-R fragment. The PphaR-R-C1-R fragment is shown in SE...
Embodiment 2
[0131] The cdc6E and crtB genes were simultaneously edited and transcriptionally regulated by the I-B CRISPR-Cas system of the HH DLCR strain of S. The crtB gene is shown in SEQ ID NO: 1 of the sequence listing. The cdc6E gene is shown in SEQ ID NO: 10 of the Sequence Listing.
[0132] 1. Construction of editing and transcriptional regulation plasmid pGKBE-DonorE-SpacerBE
[0133] 1. Preparation of DonorE-SpacerBE fragments
[0134] There is a knockout plasmid pGKBE targeting the cdc6E gene and crtB gene of the DLCR strain of S. spainii, expressing a gRNA with two spacers and containing the Donor sequence required for knocking out the crtB gene and the Donor required for knocking out the cdc6E gene sequence. The two spacers are the spacers targeting the crtB gene (S B ) and a spacer (S) targeting the cdc6E gene E ). In the gRNA expressed by plasmid pGKBE, S B The length is 36nt (positions 269-304 of the crtB gene shown in targeting sequence 1, PAM sequence is TTC), S E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com