CRISPR-Casphi-based gene editing element and application thereof
A gene editing and component technology, applied in the fields of molecular biology and bioengineering, to achieve the effect of improving efficiency and improving delivery efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0182] Example 1: CRISPR-CasΦ-based gene cleavage single AAV delivery system
[0183] 1. Adeno-associated virus construction steps
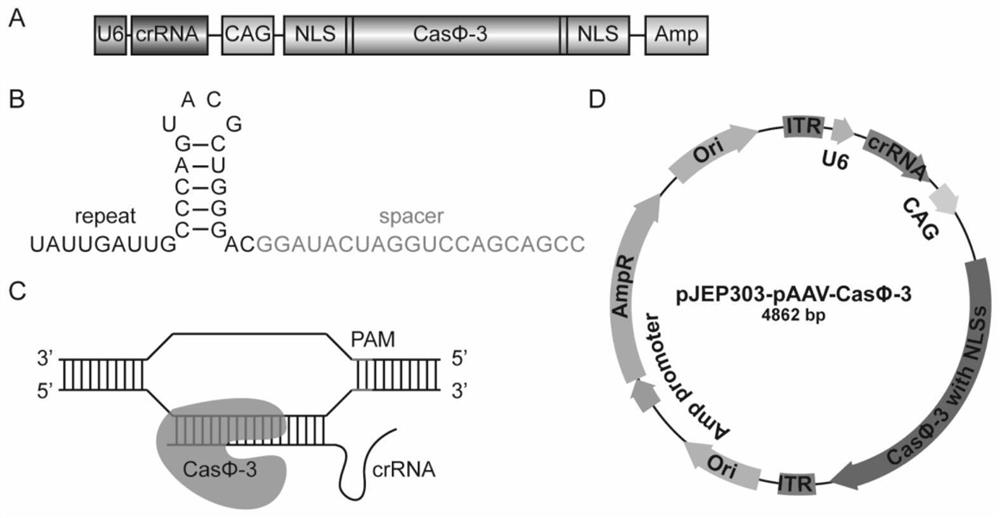
[0184] (1) According to figure 1 As shown in D, construct an adeno-associated virus plasmid (crRNA sequences are shown in SEQ ID NO: 23 and SEQ ID NO: 24);
[0185] (2) using Escherichia coli strain DH5α to amplify the adeno-associated virus vector and the adeno-associated virus backbone vector plasmid;
[0186] (3) Use lipo3000 reagent to transfect the adeno-associated virus vector and the adeno-associated virus backbone vector plasmid into 293A cells, and replace the culture medium after 6 hours;
[0187] (4) Plaque test to observe the virus state, pick the plaque with the highest titer for follow-up experiments;
[0188] (5) The virus in the medium was added to the fresh 293A cell culture medium for a small amount of virus amplification. When the cells reappeared with plaques, the cells and supernatant were collected, and the virus was co...
Embodiment 2
[0198] Example 2: CRISPRa single AAV delivery system based on CRISPR-CasΦ
[0199] 1. Adeno-associated virus construction steps
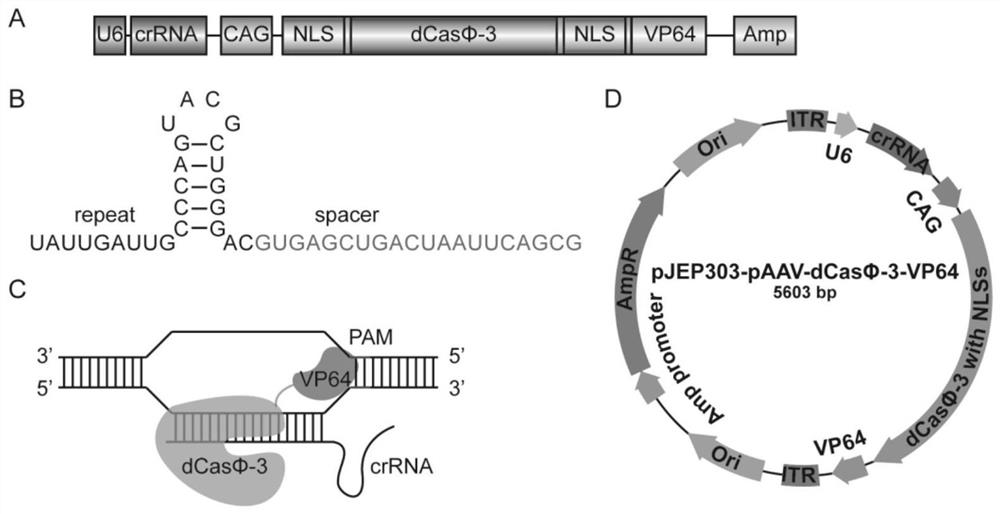
[0200] (1) According to figure 2 As shown, construct adeno-associated virus plasmid (crRNA sequences are shown in SEQ ID NO: 25 and SEQ ID NO: 26);
[0201] (2) using Escherichia coli strain DH5α to amplify the adeno-associated virus vector and the adeno-associated virus backbone vector plasmid;
[0202] (3) Use lipo3000 reagent to transfect the adeno-associated virus vector and the adeno-associated virus backbone vector plasmid into 293A cells, and replace the culture medium after 6 hours;
[0203] (4) Plaque test to observe the virus state, pick the plaque with the highest titer for follow-up experiments;
[0204] (5) The virus in the medium was added to the fresh 293A cell culture medium for a small amount of virus amplification. When the cells reappeared with plaques, the cells and supernatant were collected, and the virus was collected by ...
Embodiment 3
[0214] Example 3: CRISPRi single AAV delivery system based on CRISPR-CasΦ
[0215] 1. Adeno-associated virus construction steps
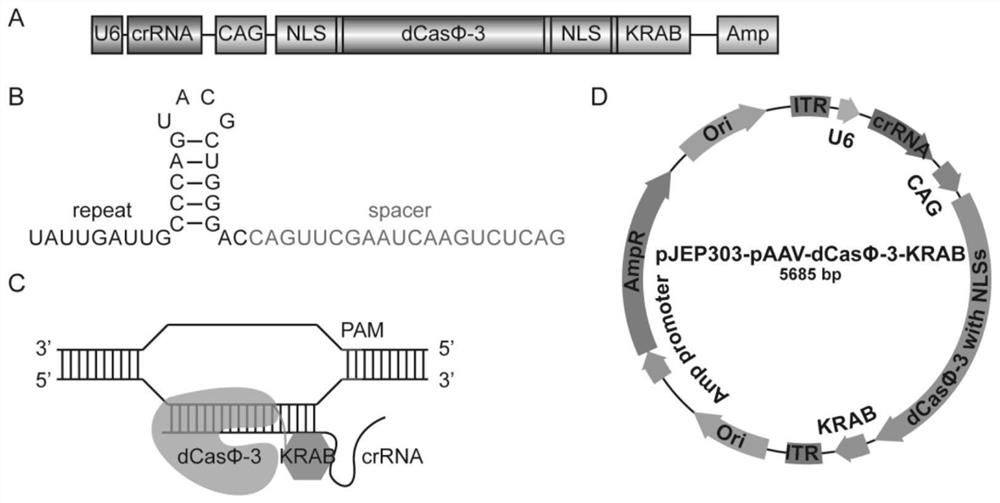
[0216] (1) According to image 3 As shown in D, construct an adeno-associated virus plasmid (crRNA sequences are shown in SEQ ID NO: 27 and SEQ ID NO: 28);
[0217] (2) using Escherichia coli strain DH5α to amplify the adeno-associated virus vector and the adeno-associated virus backbone vector plasmid;
[0218] (3) Use lipo3000 reagent to transfect the adeno-associated virus vector and the adeno-associated virus backbone vector plasmid into 293A cells, and replace the culture medium after 6 hours;
[0219] (4) Plaque test to observe the virus state, pick the plaque with the highest titer for follow-up experiments;
[0220] (5) The virus in the medium was added to the fresh 293A cell culture medium for a small amount of virus amplification. When the cells reappeared with plaques, the cells and supernatant were collected, and the virus was collec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com