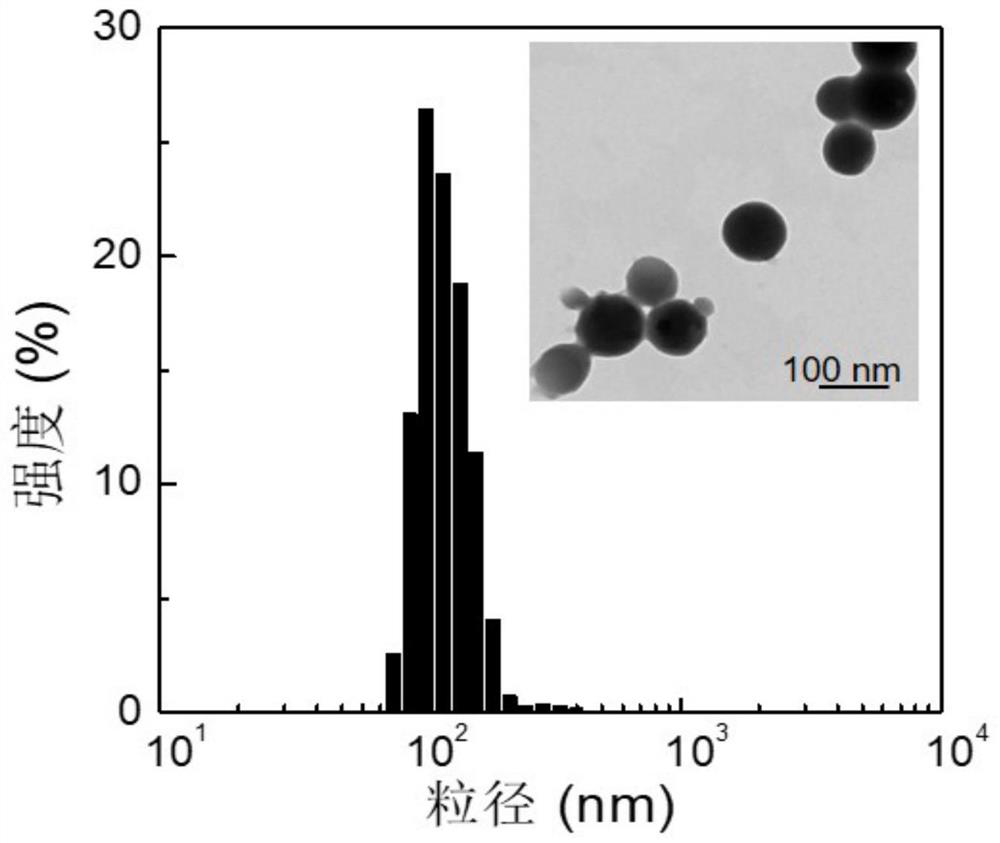
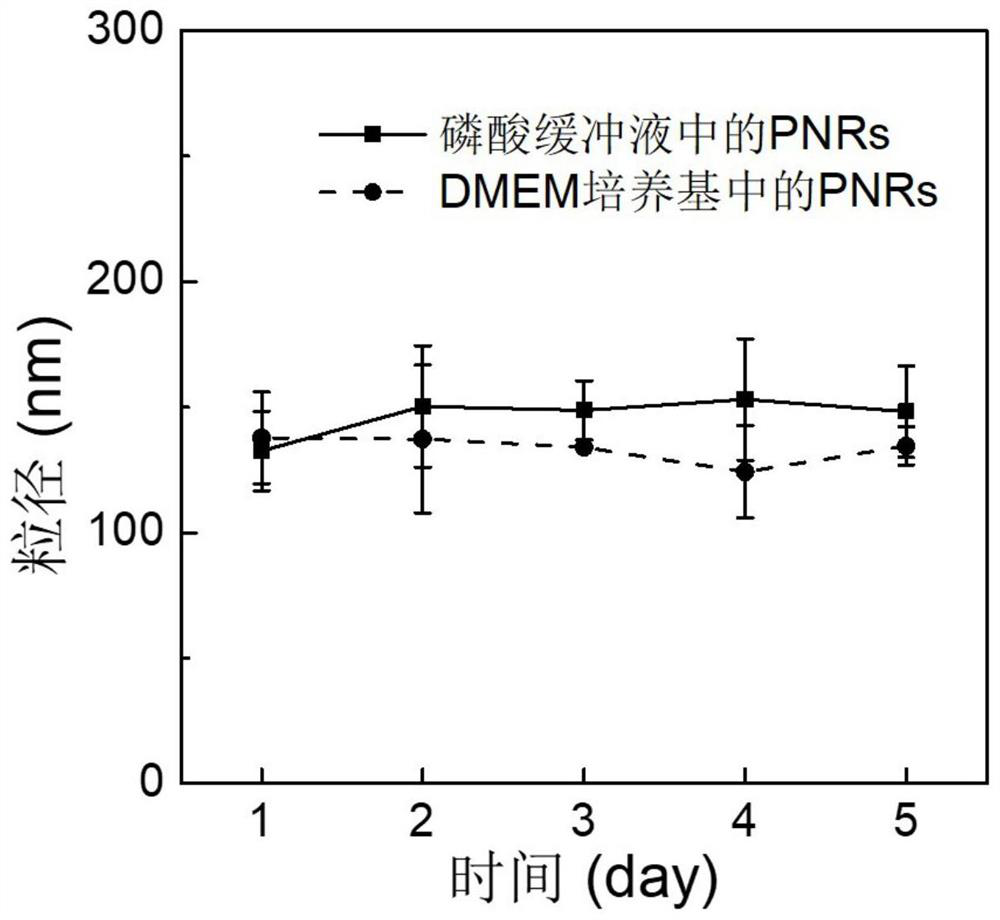
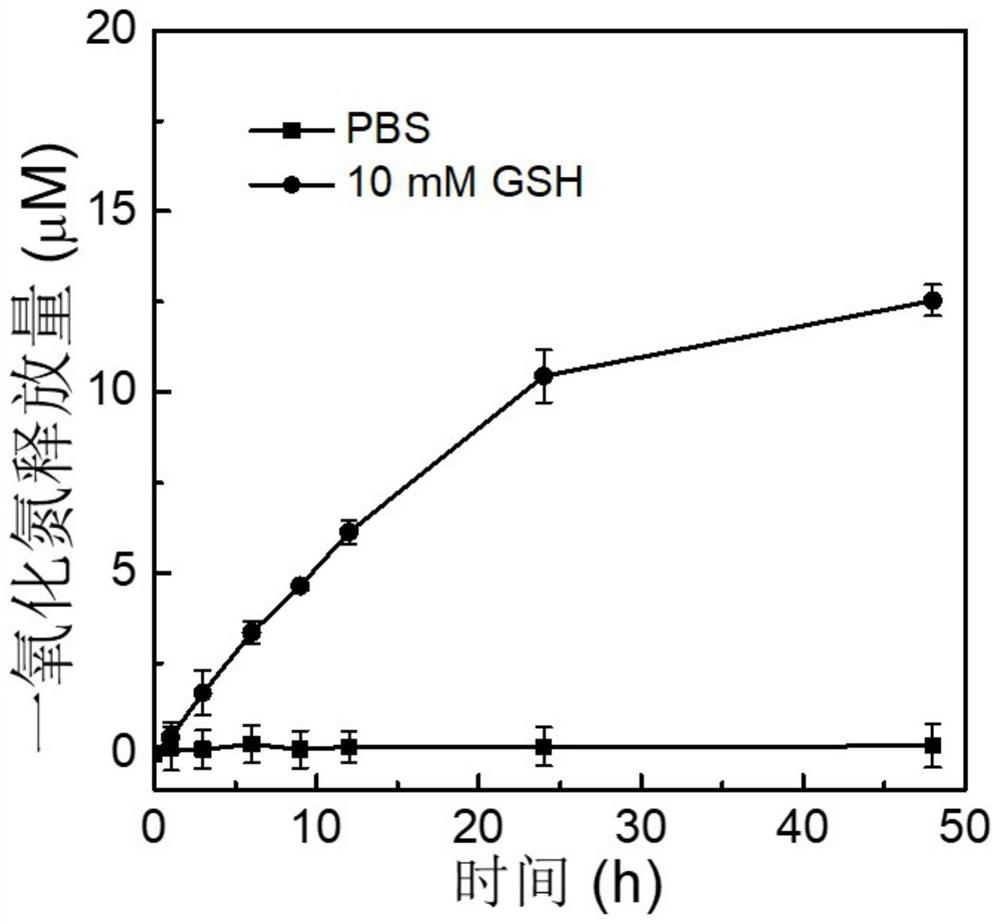
A Nitric Oxide and Cisplatin Co-delivery Polyprodrug Nanoparticles and Its Preparation and Application
A technology of nitric oxide and nanoparticles, applied in the field of medical polymers, can solve the problems of low curative effect, toxic and side effects, etc., and achieve the effect of improving drug stability, good stability, and improving anti-tumor efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of reductive response p-nitromethylstyrene monomer p-CMS-NO
[0047] In a 100 mL round-bottomed flask, weigh silver nitrate (0.934 g, 5.5 mmol), p-chloromethylstyrene (0.6 g, 3.93 mmol) and 10 mL of acetonitrile, and stir overnight at room temperature in the dark under magnetic stirring conditions. The salt was removed by filtration, the solvent was removed by rotary evaporation, then dissolved in ethyl acetate, washed three times each with water and saturated aqueous NaCl solution, the organic phase was collected, dried over anhydrous sodium sulfate, then filtered and concentrated. A yellow liquid product 1 (0.851 g, yield: 55.5%) was obtained.
[0048] The specific reaction equation is as follows:
[0049]
Embodiment 2
[0050] Example 2. Reduction-responsive cisplatin monomer PPM preparation
[0051] First, cisplatin (0.2g, 0.667mmol) was dissolved in deionized water (5mL), hydrogen peroxide (7mL6.0mmol) was added and stirred at 70°C for 5h to obtain hydroxylated cisplatin. Each was washed 3 times and dried under vacuum (78.6% yield). Dissolve the above hydroxylated cisplatin (0.2g, 0.6mmol) in 2mL of N,N-dimethylacetamide, add methacrylic anhydride (0.19g, 1.23mmol), stir at 70°C overnight, add 1mL of acetone to obtain a bright yellow solid , washed three times with acetone and ether, and dried in vacuo to obtain a yellow solid powder (0.22 g, yield: 63.5%).
[0052] The specific reaction equation is as follows:
[0053]
Embodiment 3
[0054] Example 3. Preparation of polymer p(DMA-co-Pt-co-NO) by "one-pot" method
[0055] DMA (70mg, 7.0×10 -4 mol), PPM (49mg, 1.04×10 -4 mol), p-CMS-NO (52mg, 3.0×10 - 4 mol), chain transfer agent 4-cyano-4-(phenylthiocarbonylthio)valeric acid (2.9mg, 1.03×10 -5 mol), azobisisobutyronitrile (1.7mg, 1.03×10 -5 mol), 6 mL of 1,4-dioxane and dimethyl sulfoxide (volume ratio=2:1) were added to the ampoule to dissolve. The mixture was degassed and freeze-thawed for three times, then sealed under vacuum, stirred at 65°C for 24 hours, and then stopped the polymerization reaction with liquid nitrogen, opened the reaction flask, dialyzed the reaction mixture (1.5KDa), and vacuum lyophilized to obtain soil Yellow powder.
[0056] The specific reaction equation is as follows:
[0057]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com