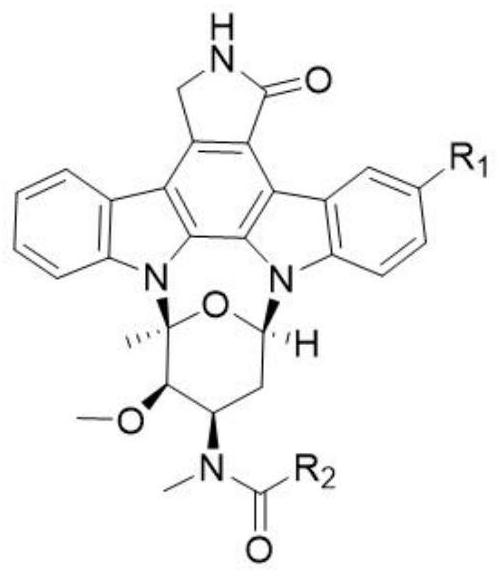
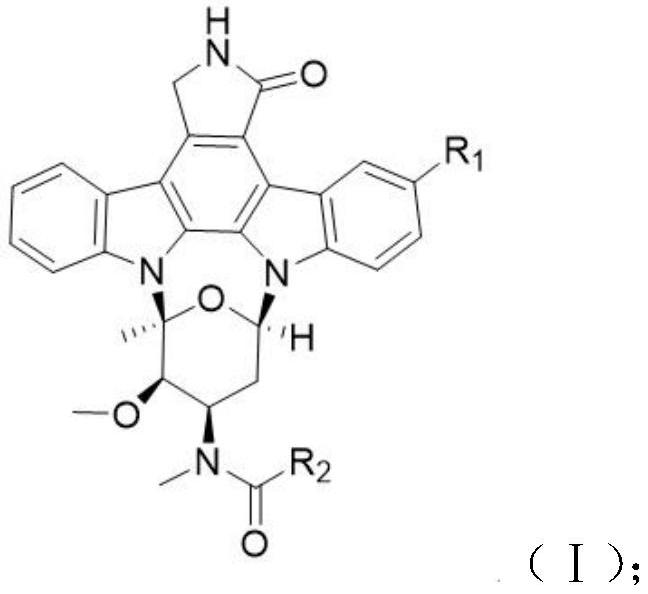
Rostaurosporine compound as well as preparation method and application thereof
A technology of staurosporine and compounds, applied in the field of staurosporine compounds and their preparation, can solve the problems of strong cytotoxicity and poor selectivity, and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
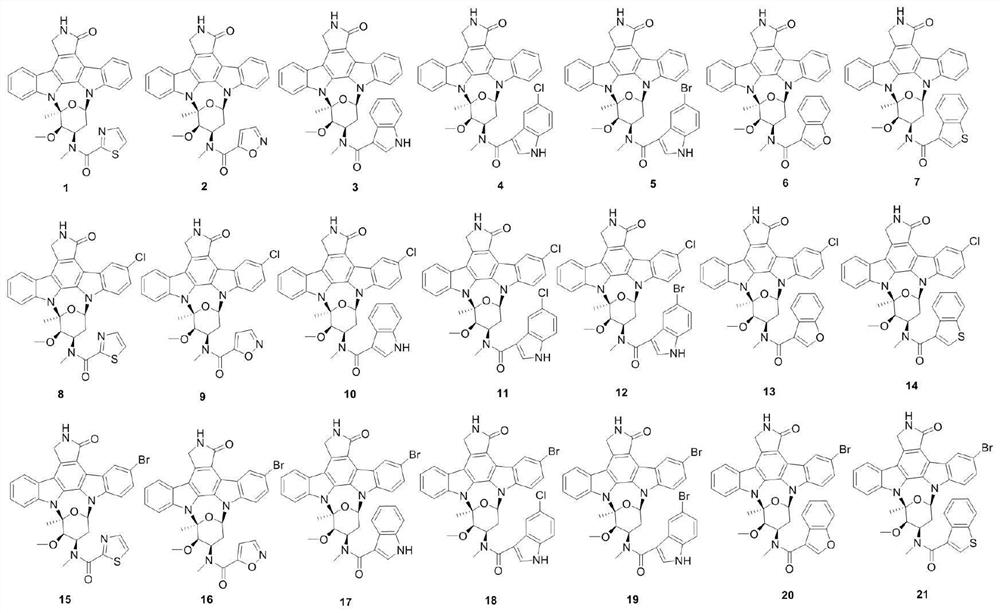
[0015] Example 1 Preparation of compound 1
[0016] Under argon protection, in a 25mL two-necked reaction flask, add 34.5mg staurosporine (0.074mmol), dissolve with 5mL dichloromethane, add 100μL triethylamine, and add 21.9mg thiazole-2-carbonyl chloride (0.148 mmol), warmed to room temperature for 2 hours, added water to terminate the reaction, extracted with dichloromethane, dried over anhydrous sodium sulfate and concentrated, separated by silica gel column chromatography, and eluted with dichloromethane:ethyl acetate (v / v 5:1) to obtain White powder 3'-N-(2-thiazolecarboxyl)staurosporine (1) (31.2 mg, yield 74.0%).
[0017] 1 H NMR (400MHz, DMSO-d 6 ): δ9.31(d, J=7.8Hz, 1H), 8.64(s, 1H), 8.24 / 8.16(s, 1H), 8.03(m, 3H), 7.63(m, 1H), 7.47(m, 2H), 7.35(t, J=7.8Hz, 1H), 7.30(m, 1H), 7.06 / 7.02(t, J=7.0Hz, 1H), 6.00 / 5.10(d, J=12.0Hz, 1H), 5.02(s, 2H), 4.65 / 4.48(s, 1H), 3.33 / 2.90(s, 3H), 2.85(m, 1H), 2.77(s, 3H), 2.42 / 2.39(s, 3H), 2.35( m,1H); 13 C NMR (100MHz, DMSO-d 6 )...
Embodiment 2
[0018] Example 2 Preparation of compound 2
[0019] Under argon protection, in a 25mL two-necked reaction flask, add 46.6mg of staurosporine (0.1mmol), dissolve with 5mL of dichloromethane, add 100μL of triethylamine, and add 26.4mg of isoxazole-5-formyl chloride at 0°C (0.2mmol), warmed to room temperature and reacted for 2 hours, added water to terminate the reaction, extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated, separated by silica gel column chromatography, washed with dichloromethane:ethyl acetate (v / v 5:1) A white powder, 3'-N-(5-isoxazolecarboxyl)staurosporine (2) (49.5 mg, yield 88.2%), was obtained.
[0020] 1 H NMR (400MHz, DMSO-d 6 ):δ9.30(d,J=8.0Hz,1H),8.94 / 8.78(s,1H),8.63(s,1H),8.07(d,J=8.0Hz,1H),8.02(d,J= 8.0Hz, 1H), 7.65 / 7.56(d, J=8.0Hz, 1H), 7.49(t, J=8.0Hz, 2H), 7.37(t, J=8.0Hz, 1H), 7.31(t, J= 8.0Hz, 1H), 7.22 / 7.03(s, 1H), 7.08 / 6.96(t, J=7.5Hz, 1H), 5.06 / 4.41(d, J=13.0Hz, 1H), 5.01(s, 2H), 4.51 / 4.46(s,1H), 2.95 / 2.87...
Embodiment 3
[0021] Example 3 Preparation of compound 3
[0022] Under argon protection, in a 100mL two-necked reaction flask, add 1.6g indole-3-carboxylic acid (10.0mmol), dissolve with 30mL dichloromethane, add 1.2g p-dimethylaminopyridine DMP (10.0mmol) and 2.2g di-tert-butyl dicarbonate (Boc 2 O) (10.0 mmol), warmed to room temperature and reacted for 1 hour, added water to stop the reaction, extracted with ethyl acetate, dried over anhydrous sodium sulfate and concentrated, separated by silica gel column chromatography, washed with petroleum ether:ethyl acetate (v / v 4:1) Detoxification gave N-tert-butoxycarbonylindole-3-carboxylic acid (1.7 g, yield 65.1%) as a white solid, ESI-MS m / z 260.0 [M–H] –. Under argon protection, in a 25mL two-necked reaction flask, add 46.6mg staurosporine (0.1mmol), dissolve it with 5mL N,N-dimethylformamide (DMF), add 100μL triethylamine, 52.2mg fresh Prepared N-tert-butoxycarbonyl indole-3-carboxylic acid (0.2 mmol), 38.4 mg (0.2 mmol) 1-(3-dimethyl...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap