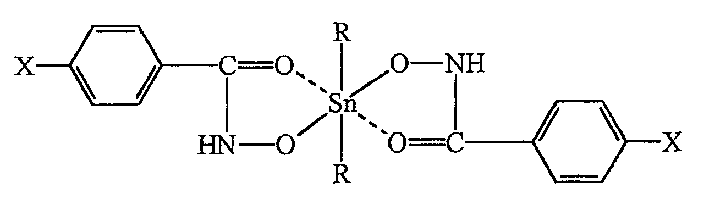
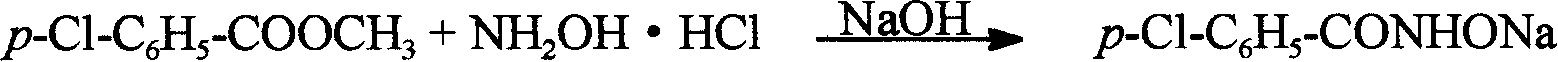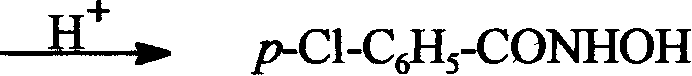Synthesis of dihydroxytin mononuclear p-chloro, p-fluoro or p-methoxyl-benzoyl hydroxamate complex
A technology of dihydrocarbyltin methoxybenzoyl hydroxamate and dihydrocarbyltin hydroxamate, which is applied in the fields of tin organic compounds and organic chemistry, and can solve unfavorable industrial production, difficulty in developing drugs, and equipment requirements advanced questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Complex [Bu n 2 Sn(p-Cl-C 6 h 5 -CONHOH) 2 ] of synthesis, the structure is as mentioned above:
[0095] 1. Synthetic route
[0096]
[0097]
[0098]
[0099] 2. Preparation method
[0100] (1) Ligand HL 1 ——p-Cl-C 6 h 5 -Synthesis of CONHOH:
[0101] Dissolve 2.5 grams (62 mmol) of sodium hydroxide in 15 ml of ice water, and slowly add 2.2 grams (32 mmol) of hydroxylamine hydrochloride in 30 ml of aqueous solution with stirring, then add 20 mmol of methyl p-chlorobenzoate (p-Cl-C 6 h 5 -COOCH 3 ), stirred at room temperature. Acidify to pH 7.5 with 5M HCl in an ice bath, a white precipitate precipitates, and is suction filtered. Recrystallize in a methanol / water mixed solvent, dry to constant weight, and obtain the product ligand HL 1 .
[0102] (2) Synthesis of mononuclear p-chlorobenzoyl hydroxamic acid dihydrocarbyl tin complex:
[0103] Will Bu n 2 SnCl 2 (0.303g 1.0mmol) added to HL 1 (0.344-g, 2.0 mmol) in KOH (0.112 g, 2...
Embodiment 2
[0107] Complex [Bu n 2 Sn(p-CH 3 O-C 6 h 5 -CONOH) 2 ] of synthesis, the structure is as mentioned above:
[0108] 1. Synthetic route
[0109]
[0110]
[0111]
[0112] 2. Preparation method
[0113] (1) Ligand (HL 3 )——p-CH 3 O-C 6 h 5 -Synthesis of CONHOH:
[0114] Dissolve 2.5 grams (62 mmol) of sodium hydroxide in 15 ml of ice water, and slowly add 2.2 grams (32 mmol) of hydroxylamine hydrochloride in 30 ml of aqueous solution with stirring, then add 20 mmol of methyl p-methoxybenzoate (p-CH 3 O-C 6 h 5 -COOCH 3 ), stirred at room temperature. Acidify to pH 7.5 with 5M HCl in an ice bath, a white precipitate precipitates, and is suction filtered. Recrystallize in a methanol / water mixed solvent, dry to constant weight, and obtain the product ligand HL 3 .
[0115] (2) Synthesis of mononuclear p-methoxybenzoyl hydroxamic acid dihydrocarbyl tin complex:
[0116] Will Bu n 2 SnCl 2 (0.576g, 1.8mmol) added to HL 3 (0.504g, 3.0mmol) i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com