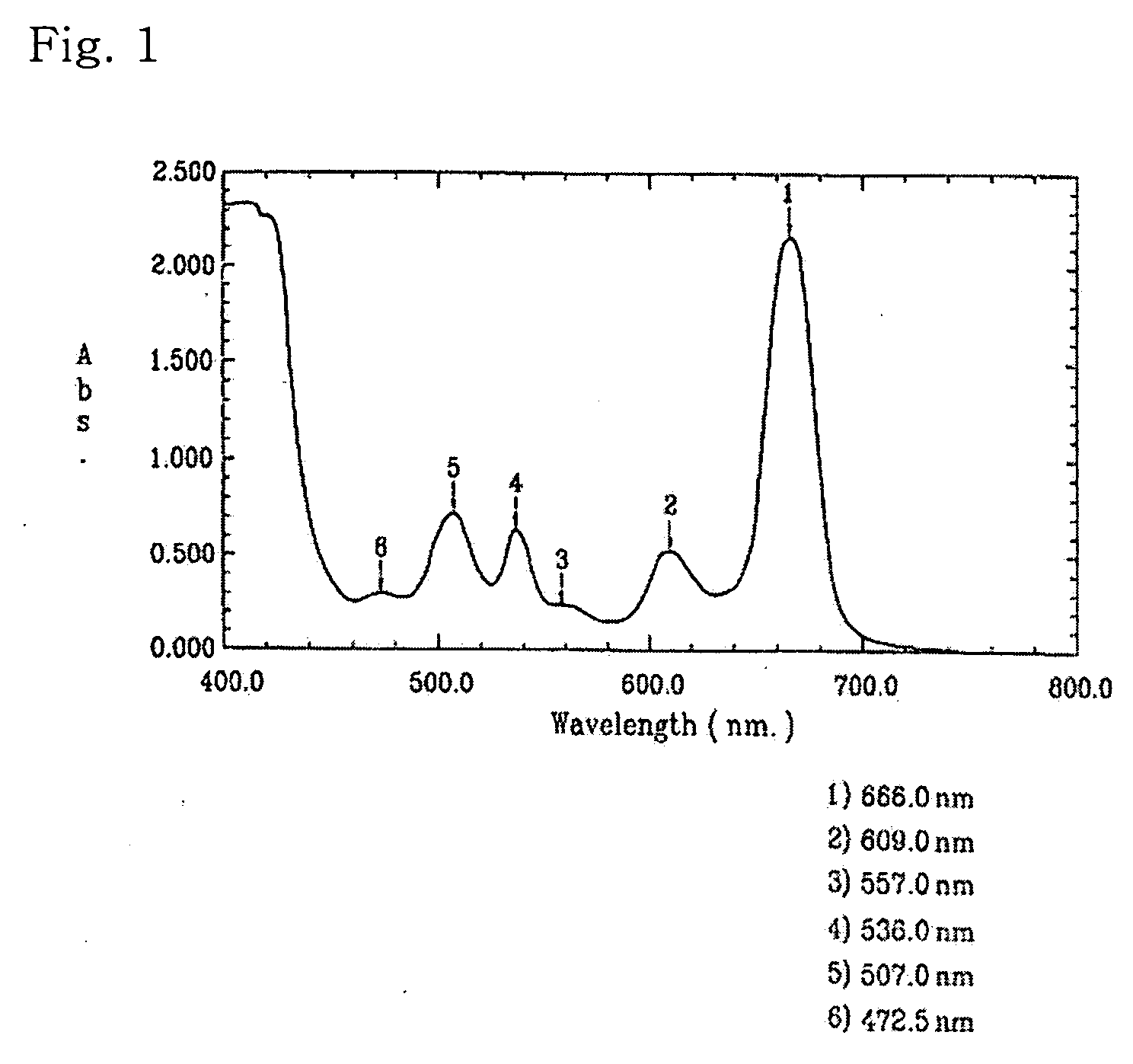
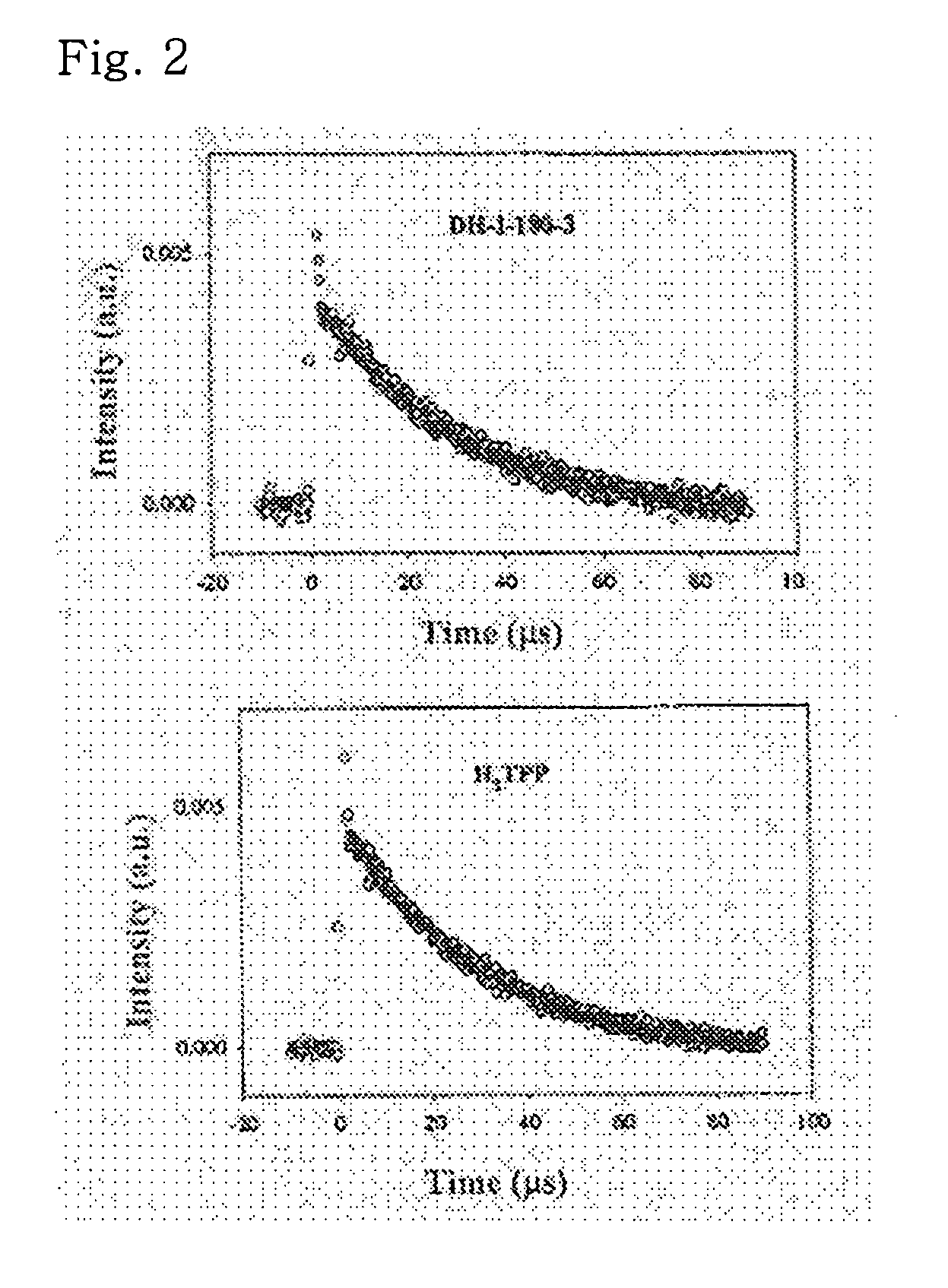
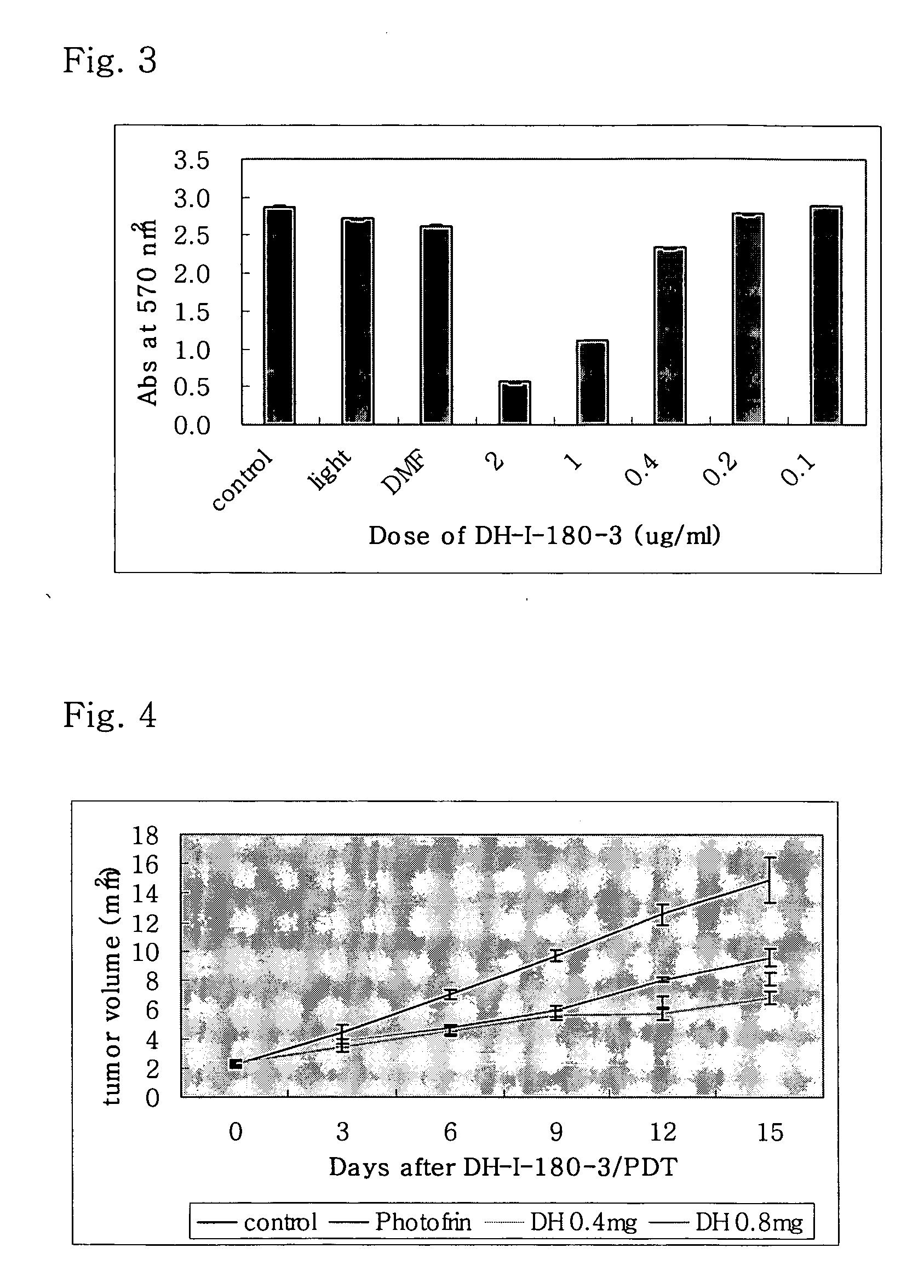
Novel use of porphyrin derivatives
a technology of porphyrin and derivatives, applied in the field of new porphyrin derivatives, to achieve the effect of superior cell cytotoxic activity and potent anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of (13-diethylene glycol-oxycarbonyl)-pheophorbide a, methyl ester (2)
[0075] A solution of phephytin a 60 mg in dichloromethane (3 ml) was poured in 50 ml of flask and treated with diethyleneglycol (20 ml) with stirring. 1 ml of sulfuric acid was added thereto, stirred for 3 hrs and then sodium bicarbonate water solution was added thereto. The solution was extracted with chloroform and the collected chloroform layer was concentrated by removing organic solvent. Remaining residue was purified by column chromatography to isolate 39 mg of (13-diethylene glycol-oxycarbonyl)-pheophorbide a, methyl ester (2):
[0076]1H-NMR (500 MHz, CDCl3) δ: 9.51 (s, 1H, meso-H), 9.37(s, 1H, meso-H), 8.56(s, 1H, meso-H), 7.99(dd, 1H, J=6.2, 11.6 Hz, CH2═CH), 6.29 (d, 1H, J=17.8 Hz, CH2═CH), 6.28(s. 1H, CH), 6.18 (d, 1H, J=11.6 Hz CH2═CH), 4.48-4.41 (m, 1H, CH), 4.24-4.22 (m. 1H. CH), 4.19-4.04 (m, 2H, OCH2), 3.87 (s, 3H, OCH3), 3.71-3.66 (m, 2H, CH2), 3.68 (s, 3H, CH3), 3.64-3.42 (m, 6H, CH2O...
example 2
Preparation of (13- methoxytriethylene glycol-oxycarbonyl) pheophorbide a methyl ester (3)
[0077] 29 mg of (13- methoxytriethylene glycol -oxycarbonyl) pheophorbide a methyl ester (3) was prepared by the same procedure with that described in above Example 1 except using phephytin (60 mg) and methoxytriethyleneglycol (30 ml):
[0078]1H-NMR (500 MHz, CDCl3) δ: 9.45 (s, 1H, meso-H), 9.30(s, 1H, meso-H), 8.55(s, 1H, meso-H), 7.93(dd, 1H, J=6.2, 11.5 Hz, CH2═CH), 6.26 (s. 1H, CH), 6.25 (d, 1H, J=17.8 Hz CH═CH2), 6.14 (d, 1H, J=11.3 Hz CH═CH2), 4.49-4.44 (m, 1H, CH), 4.22-4.20 (m. 1H. CH), 4.15-4.02 (m, 2H, OCH2), 3.88 (s, 3H, OCH3), 3.67 (s, 3H, CH3), 3.60(q, 2H, CH3—CH2), 3.51-3.45 (m, 8H, CH2 OCH2 CH2 OCH2), 3.41-3.37 (m, 2H, CH2), 3.38 (s, 3H, CH3), 3.25 (s, 3H, OCH3), 3.16 (s, 3H, CH3), 2.65-2.18 (m, 4H, CH2CH2), 1.82 (d, 3H, J=7.2 Hz, CH3), 1.68-1.64 (m, 3H, CH3), 0.51 (br. s., 1H, N-H), -1.61 (br. s., 1H, N-H).
example 3
Preparation of 13-hydroxy-(13-methoxytriethylene glycoloxy carbonyl) pheophorbide a methyl ester (4)
[0079] 22 mg of 13-hydroxy-(13- methoxytriethylene glycoloxy carbonyl) pheophorbide a methyl ester (4) was prepared by the same procedure with that described in above Example 1 except using 10-hydroxyphephytin a (60 mg) and methoxytriethyleneglycol (20 ml):
[0080]1H-NMR (500 MHz, CDCl3) δ: 9.62 (s, 1H, meso-H), 9.49(s, 1H, meso-H), 8.65(s, 1H, meso-H), 8.03(dd, 1H, J=6.3, 11.4 Hz, CH2═CH), 6.31 (d, 1H, J=17.8 Hz, CH═CH2), 6.20(d, 1H, J=11.6 Hz, CH═CH2), 5.78 (s, 1H, OH), 4.52-4.47 (m, 1H, CH), 4.30-4.14 (m, 3H, CH and OCH2), 3.74 (s, 3H, OCH3), 3.74-3.70(m, 2H, CH2), 3.63-3.57 (m, 8H, CH2OCH2CH2OCH2), 3.60 (s, 3H, CH3), 3.47-3.46 (m, 2H, CH2), 3.43 (s, 3H, CH3), 3.29 (s, 3H, CH3), 3.27 (s, 3H, OCH3), 3.02-2.95, 2.64-2.57 and 2.35-2.21 (m, 4H, CH2CH2), 1.71 (t, 3H, J=7.5 Hz, CH3), 1.60 (d, 3H, J=7.1 Hz, CH3), 0.30 (br. s., 1H, N-H), -1.83 (br. s., 1H, N-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| mean body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com