Tetrahydroquinoline derivatives
A technology of tetrahydroquinoline and its derivatives, applied in the field of tetrahydroquinoline derivatives or their salts, can solve the problems of no compound being created, central nervous system and reproductive system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
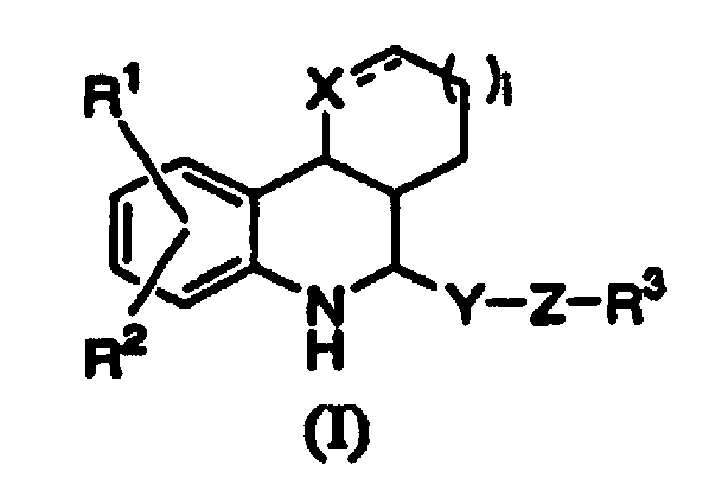
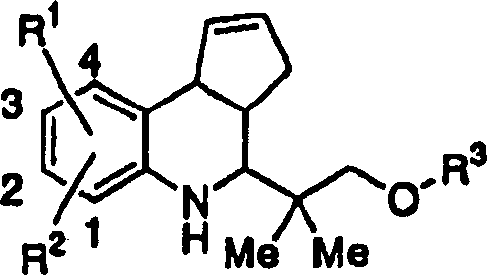
[0016] The substituents in the aforementioned formula (I) will be described below.
[0017]Specific examples of the "alkyl group having 1 to 9 carbon atoms" include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, sec-butyl, n-pentyl Base, tert-pentyl, 3-methylbutyl, neopentyl, n-hexyl, 3,3-dimethylbutyl, 2-ethylbutyl, n-heptyl, 2-methylhexyl, n-octyl , 2-propylpentyl and n-nonyl and other straight-chain or branched-chain alkyl groups.
[0018] Specific examples of the "alkoxy group having 1 to 9 carbon atoms" include methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, tert-butoxy Base, sec-butoxy, n-pentyloxy, tert-pentyloxy, 3-methylbutoxy, neopentyloxy, n-hexyloxy, 3,3-dimethylbutoxy, 2-ethylbutyl Linear or branched alkoxy groups such as oxy, n-heptyloxy, 2-methylhexyloxy, n-octyloxy, 2-propylpentyloxy and n-nonyloxy.
[0019] Specific examples of the "halogen atom" include a fluorine atom, a chlorine atom, a bromine atom, and an iodine atom. ...
Embodiment 1
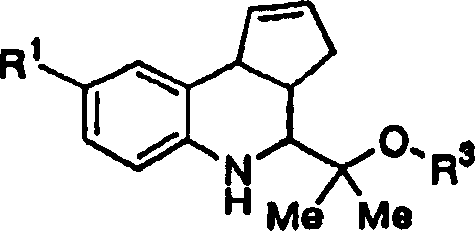
[0084] 9.8 g of 4-nitroaniline, 6.5 ml of cyclopentadiene and 5.5 ml of trifluoroacetic acid were dissolved in 70 ml of acetonitrile, and 10.0 g of hydroxytrimethylacetaldehyde was added at 0°C. After stirring at room temperature for 30 minutes, the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography (elution solvent: hexane:ethyl acetate=1:1) to obtain 4.8 g of the title compound. Physical data are shown below.
[0085] 1 H-NMR (CDCl 3 )δ values: 7.85 (s, 1H), 7.84 (d, J = 8.9Hz, 1H), 6.47 (d, J = 8.9Hz, 1H), 5.96 (brs, 1H), 5.78 (brs, 1H), 3.98 (d, J=9.9Hz, 1H), 3.64(d, J=10.6Hz, 1H), 3.55(d, J=10.6Hz, 1H), 3.54(d, J=2.3Hz, 1H), 2.87(ddt , J=2.3, 8.2, 9.9Hz, 1H), 2.48(dd, J=9.9, 15.5Hz, 1H), 2.26(dd, J=8.2, 15.5Hz, 1H), 1.11(s, 3H), 0.96( s, 3H).
[0086] Compounds shown in Examples 2 to 34 were produced in the same manner as in Example 1. The physical data of the obtained compound are shown in Tables 1-4.
...
Embodiment 50
[0101] 1 H-NMR (CDCl 3 )δ values: 8.01 (brs, 1H), 7.81-7.77 (m, 2H), 7.45 (s, 1H), 6.90 (d, J=9.9Hz, 1H), 6.38 (brs, 1H), 5.97-5.95 ( m, 1H), 5.74-5.72(m, 1H), 4.00(d, J=8.6Hz, 1H), 3.52(brs, 1H), 3.47(dd, J=7.3, 14.2Hz, 1H), 2.47-2.37 (m, 1H), 2.27-2.22 (m, 1H), 1.24 (s, 3H), 1.17 (s, 3H). [Example 50] 2-(8-nitro-3a, 4, 5, 9b- Preparation of tetrahydro-3H-cyclopentadieno[c]quinolin-4-yl)-propylamine
[0102] Using 65 mg of the compound of Example 24, the same procedure as in Example 49 was carried out to obtain 32 mg of the title compound. Its physical data are shown below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com