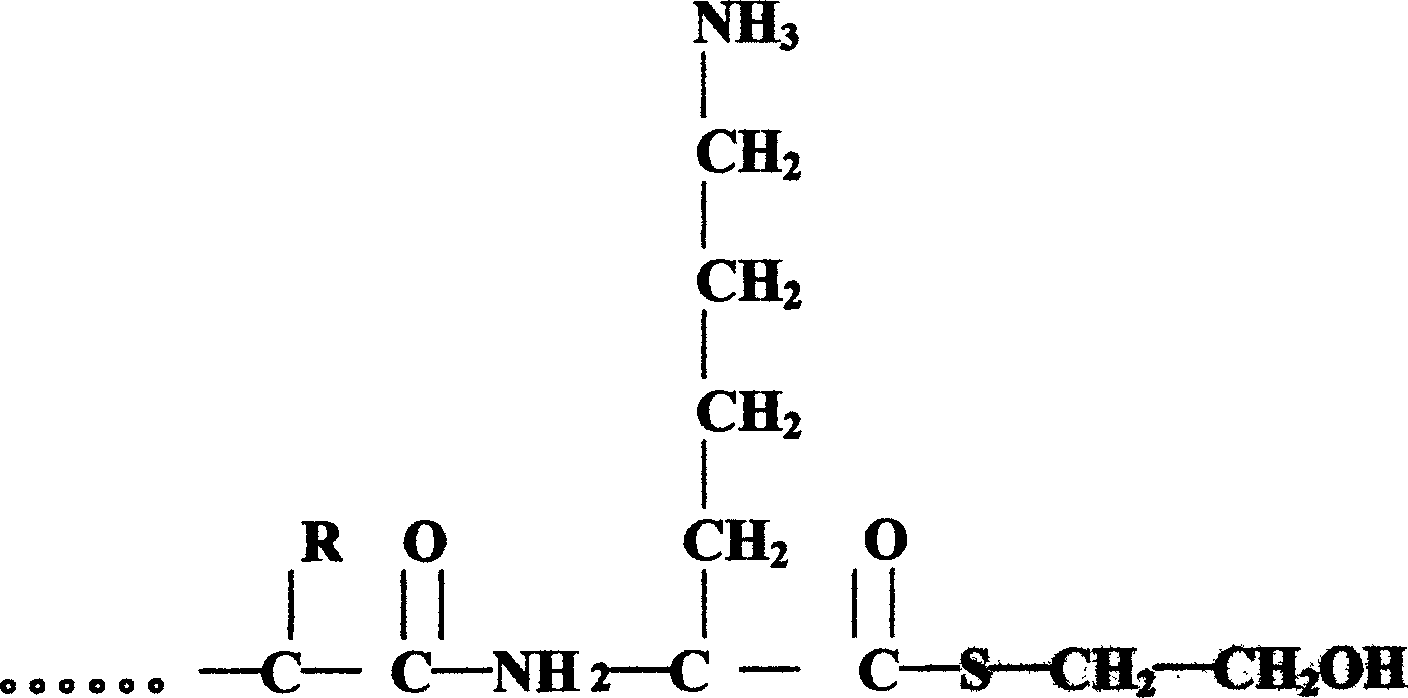
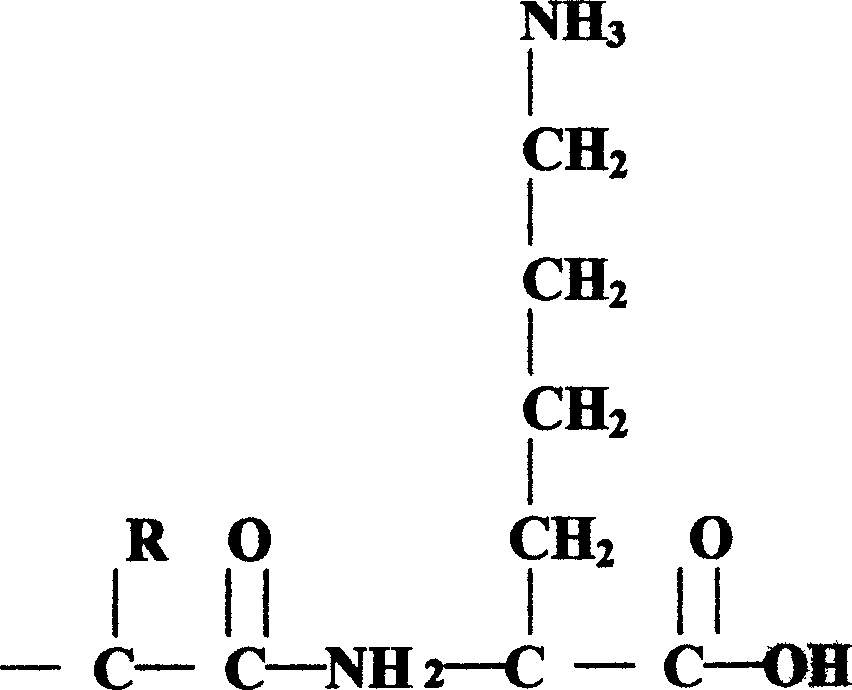
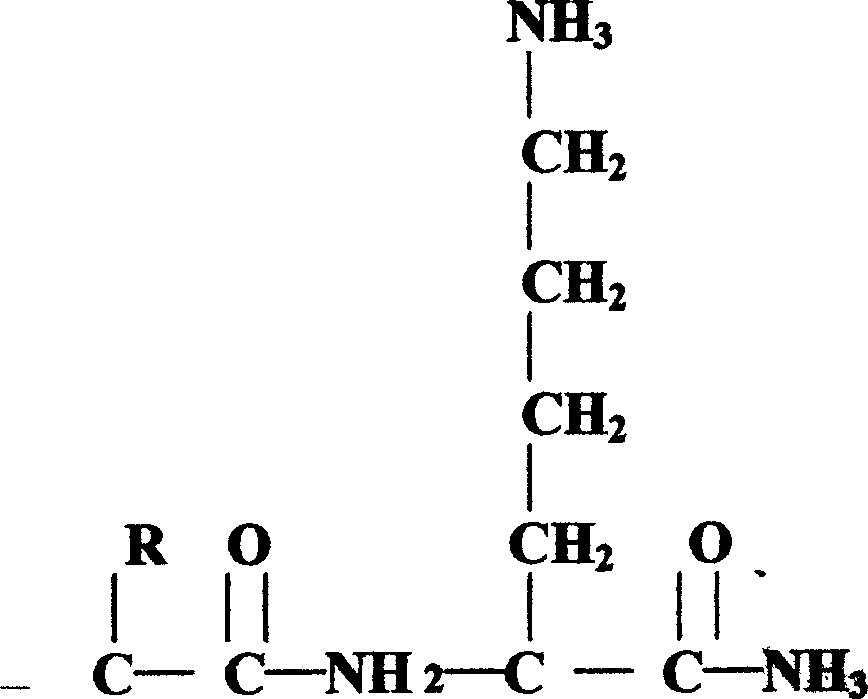
Pituitary adenylate cyclase activated polypeptide derivatives and process for preparing the same
A technology of adenylate cyclase and peptide derivatives, applied in the field of genetic engineering, can solve the problems of low harvest rate and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The amplification of embodiment 1PACAP gene and the construction of PTY-PDT plasmid
[0036] According to the amino acid sequence of PACAP mature peptide, its nucleotide sequence was designed according to the codon preference of Escherichia coli. Segmented synthetic primers, according to image 3 As shown, the PACAP gene was synthesized in vitro by PCR. Three primers for the synthesis of PACAP:
[0037] F1 5'ATG CAC TCT GAC GGC ATC TTC ACA GAC TCT TAT TCC CGCTAC CGA AAA CAA ATG3';
[0038]F2 5'TCC CGC TAC CGA AAA CAA ATG GCT GTC AAG AAA TAC TTGGCG GCC GTG CTA GGG AAA AGG3';
[0039] F3 5’TTA CTA TTT GTT TTT AAC CCT CTG TTT ATA CCT TTT CCC TAGCAC GGC CGC3’
[0040] For the construction of expression fusion protein plasmid, see Figure 8 . Primers were designed to introduce the Ndel recognition site upstream of the PACAP gene and the Sapl recognition site downstream; the PACAP gene was inserted into PTYB1 to obtain the expression vector PTY-PDT. For PCR identificati...
Embodiment 2
[0041] Embodiment 2 Expression of recombinant fusion protein and cleavage of target protein
[0042] Transform the expression plasmid into the expression host strain E.coli Strain ER2566, pick a single clone and culture it overnight, inoculate it at 1:20 in 1L LB medium containing 50 mg / L kanamycin, and shake it at 37°C until the OD is 0.5- 0.8, add IPTG to a final concentration of 0.3-0.5mmol / L, and induce at 30°C for 3h. The cells were collected by centrifugation, resuspended in BufferA (20mM Tris.HCl, 500mM NaCl, 1mM EDTA, pH7.5), ultrasonically disrupted at 4°C, and centrifuged to obtain the supernatant.
[0043] Take 6mL chitin beads slurry and equilibrate with 60mL Buffer A after loading into the column; load the broken supernatant of the bacteria at a flow rate of Figure 11 , westernblot identification results see Figure 12 , mass spectrometry results see Figure 13 .
Embodiment 3
[0044] Activity and stability comparison of embodiment 3PDT and PACAP38
[0045] After the S1990 cells in the logarithmic growth phase were digested and detached, the concentration of the cell suspension was adjusted to 1×10 7 each / mL, add the same concentration of PDT and PACAP38 respectively, and use the culture medium as the control group; after incubation for 5 minutes, take out 200 μL suspension from the experimental group and the control group, add trichloroacetic acid, shake and mix well, centrifuge, and take the upper Clear 0.5 mL, wash the supernatant with water-saturated ether, and evaporate to dryness. Cyclic AMP Enzyme Immunoassay Kit was used to determine cAMP concentration.
[0046] The activity data of PDT and PACAP38 promoting cAmp production in S1990 pancreas cells by the above method are shown in Table 1, and the graph is shown in Figure 14 , the results showed that the activities of PDT and PACAP38 were comparable.
[0047] Tabl...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap