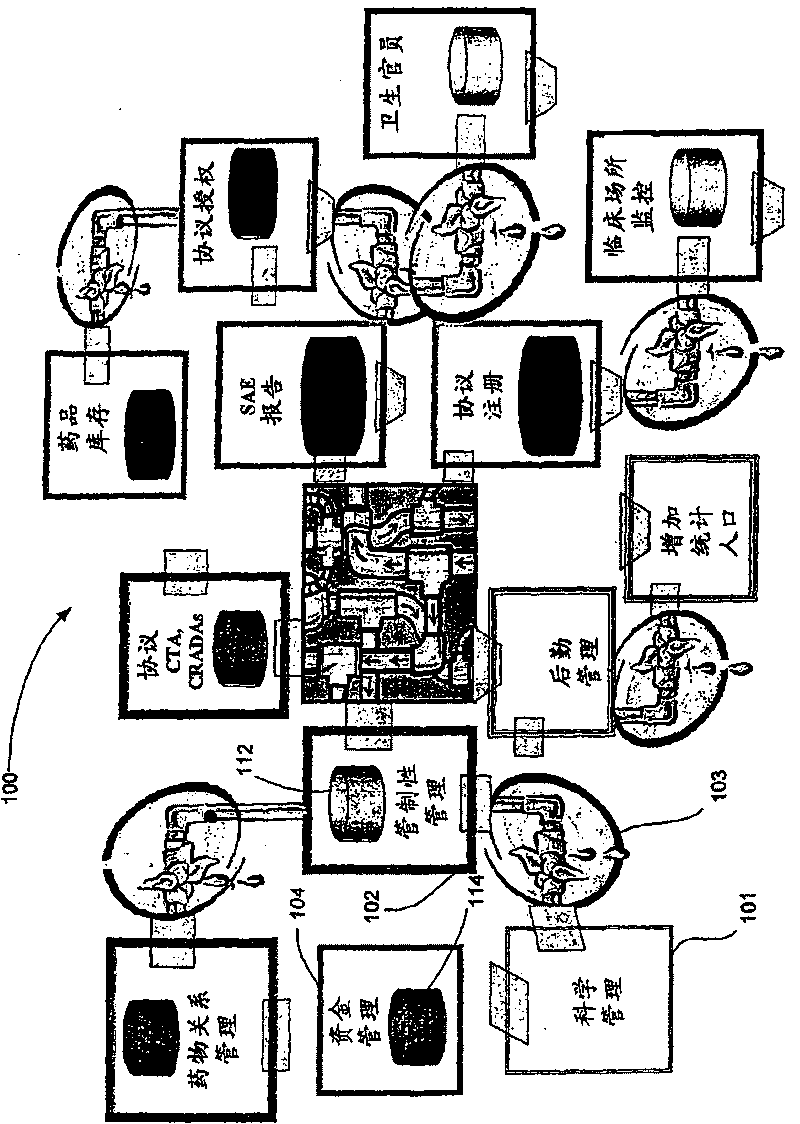
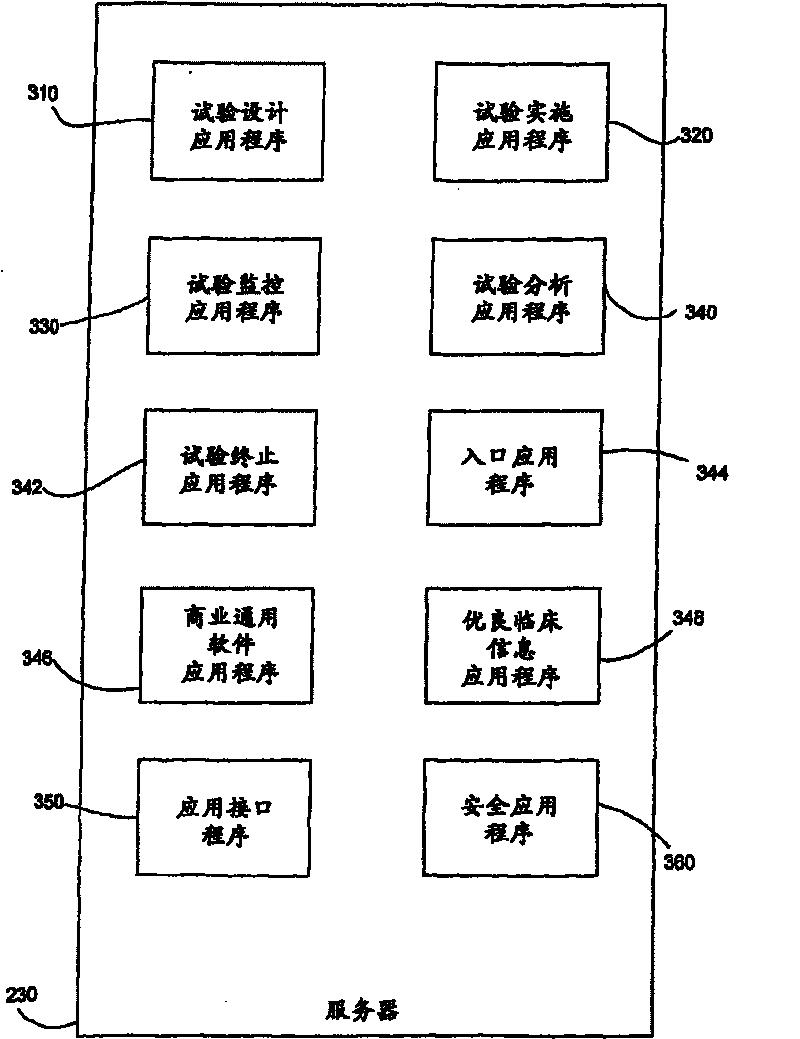
Systems and methods for clinical trials information management
A technology for clinical trials and clinical information, applied in electronic clinical trials, informatics, medical informatics, etc., can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
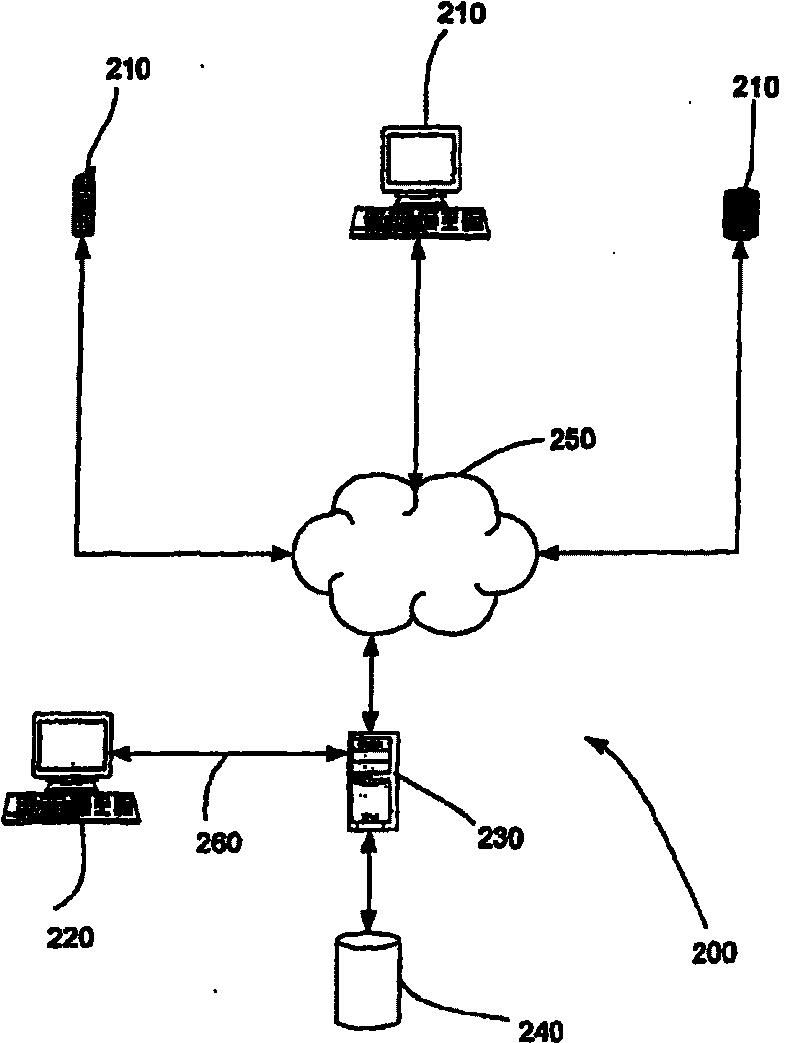
[0067] figure 2 The schematic diagram shows an exemplary system for managing clinical trials according to an embodiment of the present invention. The system 200 includes a web client 210, a client 220, and a server 230. And the database 240. The web client 210 accesses the web via a web browser. Web clients include but are not limited to computers, cellular phones, and PDAs. The web client 210 and the client 220 access the server 230. The web client 210 accesses the server 230 via the web connection 250. Those skilled in the art will understand that Web connections include intranets, extranets, and the Internet. The client 220 accesses the server via another connection 260 other than the Web connection. Those skilled in the art will understand that the connection 260 may be any connection other than a connection on which Web technology is used. This includes but is not limited to Internet connections, Ethernet connections, and application programming interfaces on the sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com