Drugs containing galectin 9
A technology of galectin and medicine, applied in the field of medicine containing galectin 9
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0164] (1) Preparation of immunogenic antigen, (2) Immunization of animals with immunogenic antigen, (3) Preparation of myeloma cells, (4) Cell fusion of antibody-producing cells and myeloma cells, (5) Hybridoma ( fused cells) selection and monocloning, and (6) production of monoclonal antibodies
[0165] (1) Preparation of immunogenic antigen was carried out as follows. As the antigen, as described above, polypeptides isolated from natural galectin-9 polypeptides or large fragments derived therefrom (may include part of domain polypeptides, linker polypeptides, fragments, part of peptides, and synthetic polypeptides) can be used. Based on the determined amino acid sequence information of galectin 9, an appropriate oligopeptide was chemically synthesized as an antigen. Representative antigens are selected from (1) the amino acid sequence of sequence number: 1 and sequence number: 2 in the sequence listing published by WO02 / 37114 A1 or the amino acid sequence of a part of its ...
Embodiment 1
[0247] (1) Materials and methods
[0248] (a) cell culture
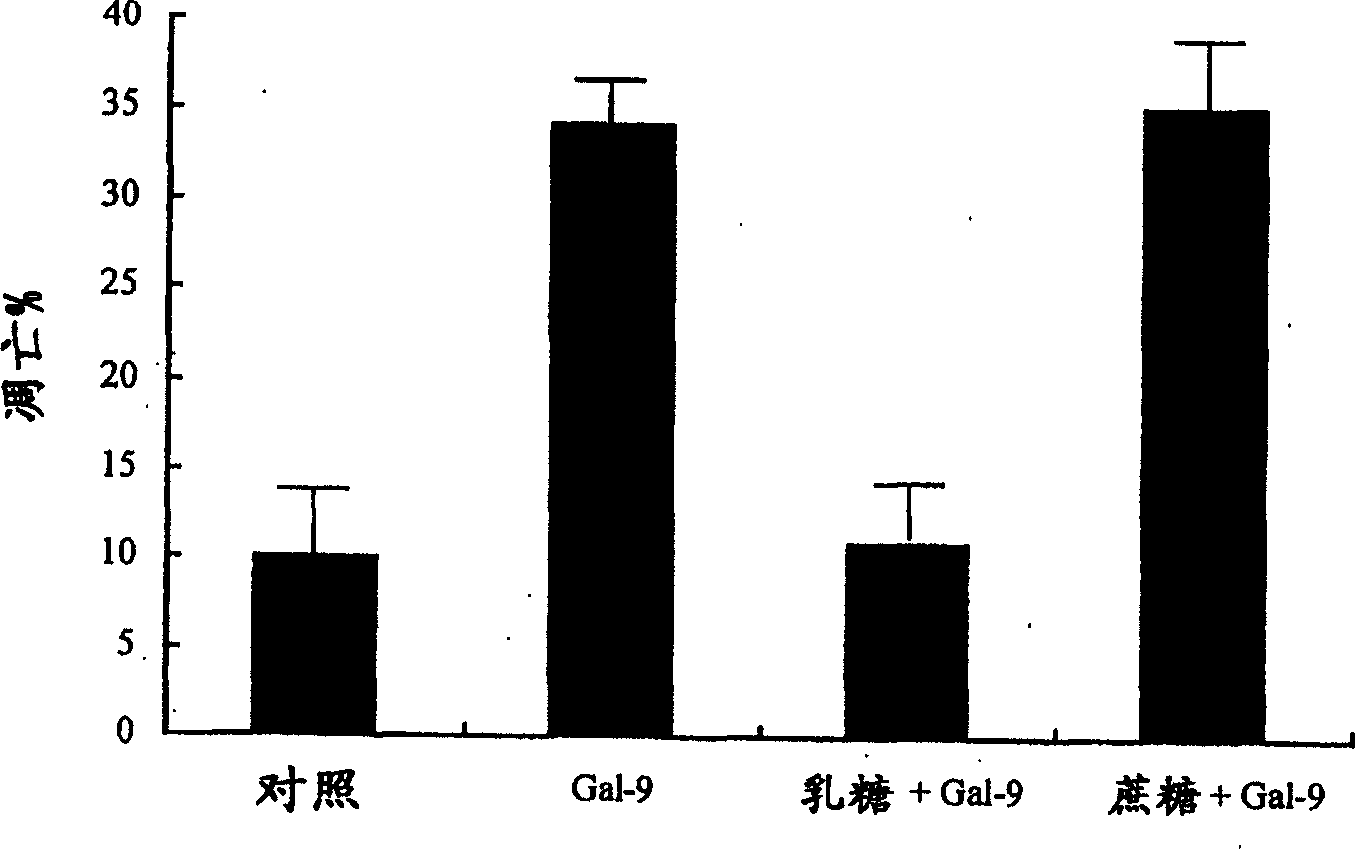
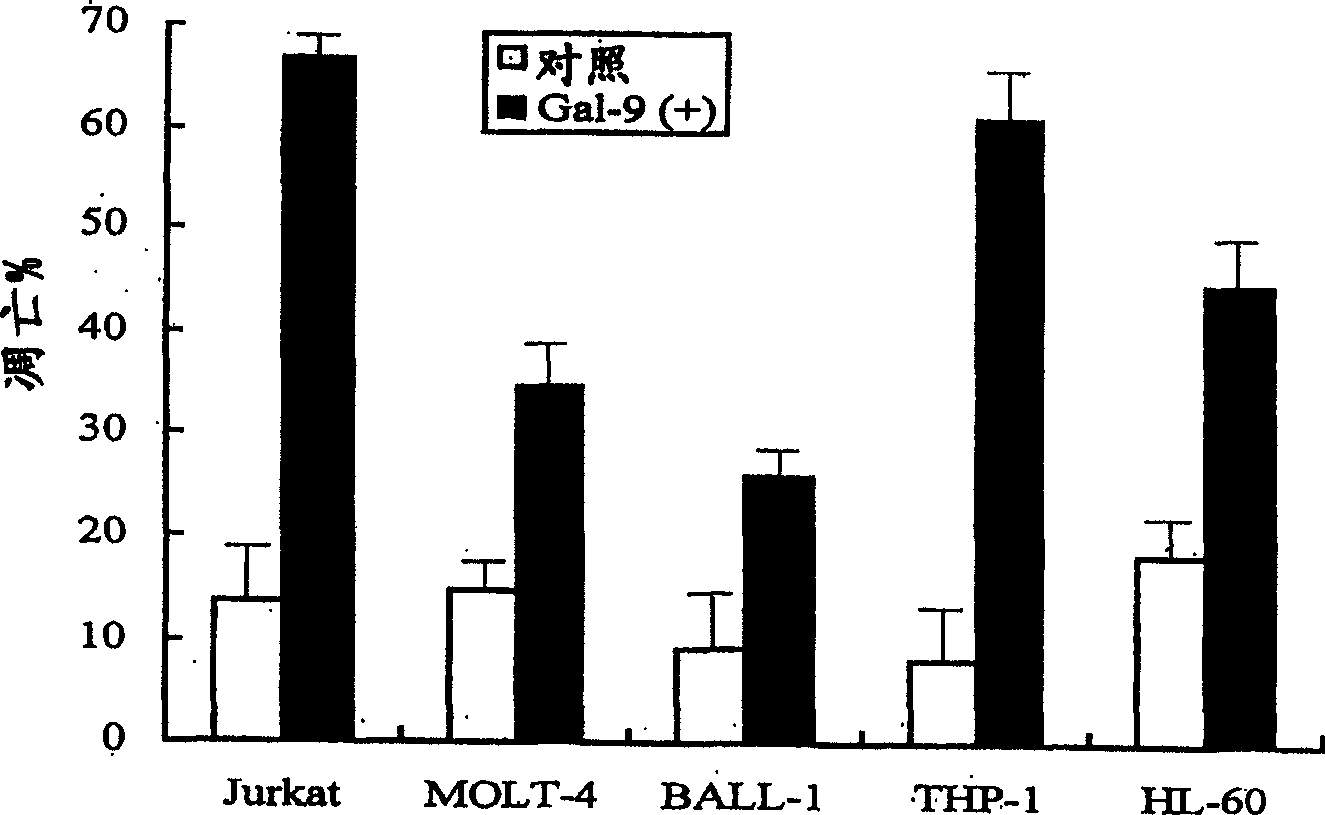
[0249] MOLT-4 (T cells), Jurkat (T cells), BALL-1 (B cells), THP-1 (cells from acute monocytic leukemia) and HL60 (cells from acute monocytic leukemia) were provided by the American Type Culture Collection ( ATCC) obtained. All cell lines were grown in RPMI-1640 medium (Sigma, St. Louis, USA) supplemented with 10% FCS at 5% CO 2 maintained at 37°C. In order to inhibit the activity of Gal-9, 30 mM lactose was added to the culture medium. The same concentration of sucrose was used as a control.
[0250] (b) Expression and purification of recombinant Gal-9 (rGal-9)
[0251] In accordance with well-known methods (for example, Matsushita, N. et al., J. Biol. Chem., 275: 8355 (2000); and Nishi, N. et al., Endocrinology, 141: 3194 (2000)), Make rGal-9 into (His) 6 - Galectin-9 (short form) [(His) 6 -Gal-9(S)], expressed and purified. That is, Escherichia coli BL-21 strain carrying a Gal-9 expression plasmid was gro...
Embodiment 2
[0306] (1) Cytotoxic activity of galectin-9
[0307] Cytotoxic activity was measured for the Jurkat T cell line, K-562 leukemia cells, and normal lymphocytes by flow cytometry analysis using PI. That is, after 16 hours of incubation of target cells with 1 mM galectin-9, PI was added for the last 15 minutes, and PI entered the damaged target cells. The fluorescence emitted by the PI was measured by FACS. Use cells without any other substances as a negative control, use formalin-fixed cells as 100% dead cells, and use H as a positive control 2 o 2 (hydrogen peroxide solution). As shown in Table 2, galectin-9 exhibited obvious cytotoxic activity on Jurkat cell line and K-562 cells, but did not exhibit harmful activity on normal lymphocytes.
[0308] Stimulate
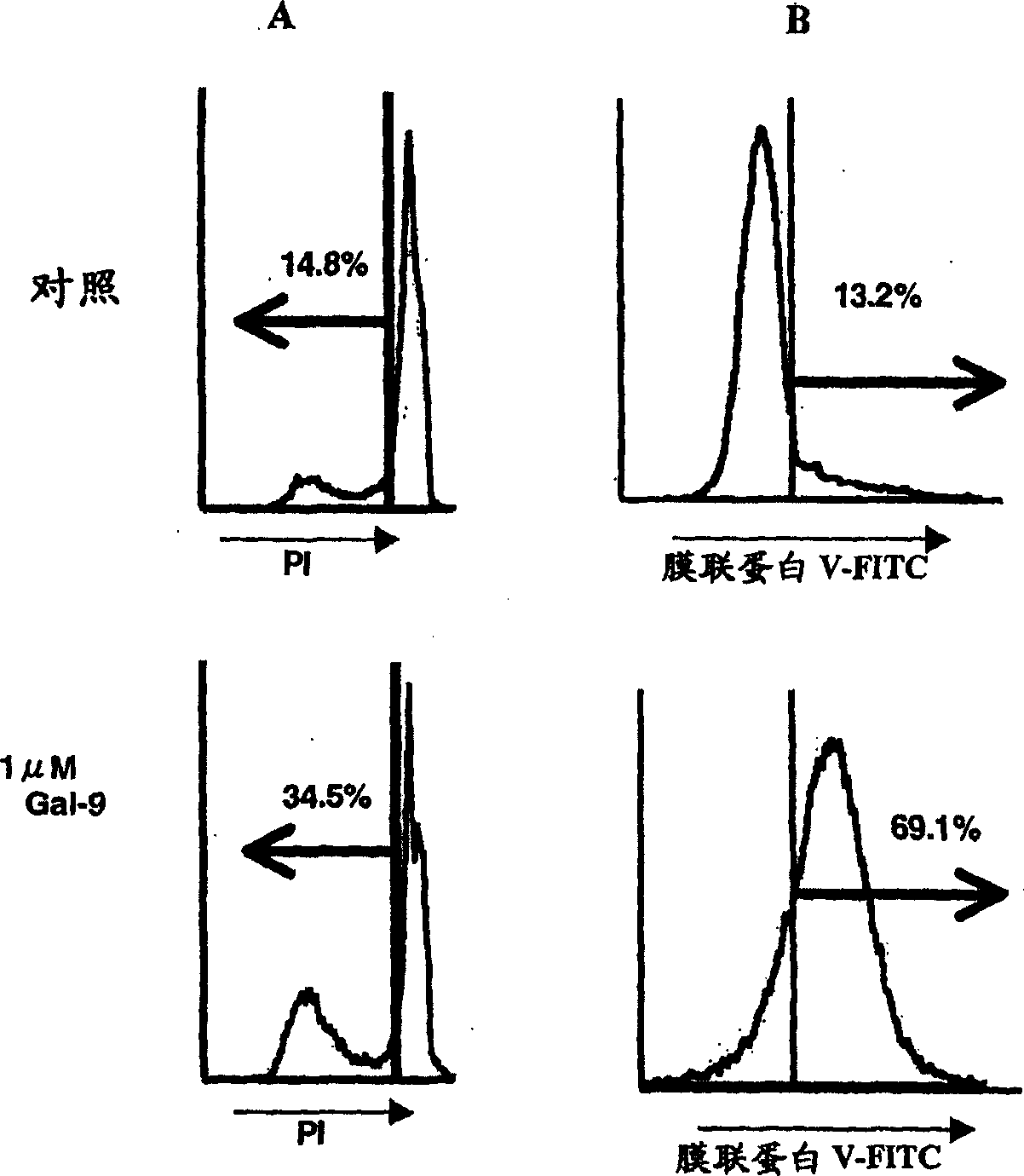
[0309] (2) Cancer cell apoptosis induced by galectin-9
[0310] Apoptosis was measured by the PI method. After the cells cultured with galectin-9 were washed, they were fixed with ethanol, and the fragme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com