Aldfuwei ester injection having liver target and its preparation method
A technology of adefovir dipivoxil and dipivoxil injection, which is applied in the field of medicine and can solve the problem of lack of liver targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
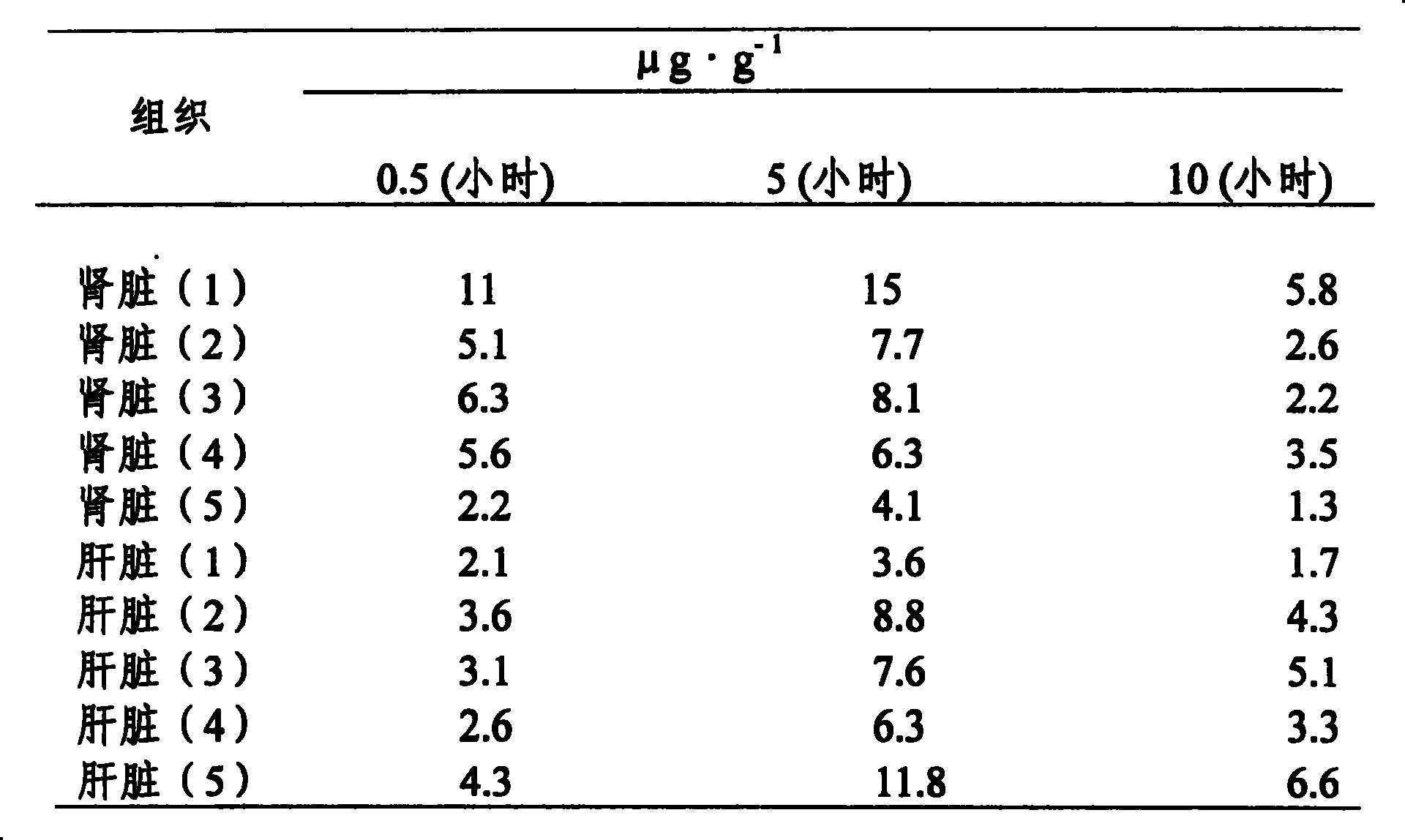
Image
Examples
Embodiment 1
[0014] Embodiment 1 Adefovir dipivoxil liposome
[0015] Component Weight (g)
[0016] Adefovir dipivoxil 1
[0017] Soy Lecithin 5
[0018] Proper amount of ethanol
[0019] citrate buffer to 500
[0020] Preparation Process:
[0021] Weigh the prescribed amount of adefovir dipivoxil and soybean lecithin, dissolve with an appropriate amount of ethanol, add citric acid buffer (0.1mol / L, pH4.0), stir and hydrate at 40°C, pass through a homogenizer (or Microjet) (the first step is to adjust the homogenization pressure to 520-600kg / cm 2 , the second step and then adjusted to 100 ~ 140kg / cm 2. Microfluidizer, the first step is to adjust the homogenization pressure to 4000-8000psi, and the second step is to adjust to 10000-16000psi) to obtain adefovir dipivoxil liposomes, the particle size of which is 20nm~300nm. The obtained liposome liquid can be added with 2% (W / V) trehalose for freeze-drying to obtain freeze-dried liposome. After reconstitution, its particle size is 10...
Embodiment 2
[0022] Embodiment 2 Adefovir dipivoxil liposome
[0023] Component Weight (g)
[0024] Adefovir dipivoxil 1
[0025] Soy Lecithin 20
[0026] Proper amount of ethanol
[0027] Alpha-tocopherol 0.1
[0028] Phosphate buffer to 1000
[0029] Preparation Process:
[0030] Weigh the prescribed amount of adefovir dipivoxil, soybean lecithin, and α-tocopherol, dissolve them with an appropriate amount of ethanol, add phosphate buffer (0.01mol / L, pH5.0), stir and hydrate at 40°C, and pass Homogenizer (or micro jet), the first step is to adjust the homogenization pressure to 520kg / cm 2 , the second step and then adjusted to 100kg / cm 2 (or microfluidizer, the first step is to adjust the homogenization pressure to 4000psi, and the second step is to adjust to 10000psi) to obtain adefovir dipivoxil liposomes, the particle size of which is 55nm~580nm. Gained liposome liquid can add the mannitol of 6% (W / V) and the glucose of 1% (W / V) and carry out freeze-drying, obtain freeze-dried ...
Embodiment 3
[0031] Embodiment 3 adefovir dipivoxil liposome
[0032] Component Weight (g)
[0033] Adefovir dipivoxil 1
[0034] Yolk Lecithin 30
[0035] Cholesterol 1
[0036] Alpha Lipoic Acid 0.5
[0037] Proper amount of ethanol
[0038] Acetate buffer up to 1000
[0039] Preparation Process:
[0040] Weigh the prescribed amount of adefovir dipivoxil, egg yolk lecithin, cholesterol, α-lipoic acid, after dissolving with appropriate amount of ethanol, adopt the ethanol injection method to add in the phosphate buffer (0.03mol / L, pH6.0), 50 Stir and hydrate at ℃, pass through a homogenizer (or micro jet), and adjust the homogenization pressure to 600kg / cm in the first step 2 , the second step and then adjusted to 100kg / cm 2 (or microfluidizer, the first step is to adjust the homogenization pressure to 5000psi, and the second step is to adjust to 10000psi) to obtain adefovir dipivoxil liposomes, the particle size is 58nm~510nm, and the encapsulation efficiency is greater than 80%....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com