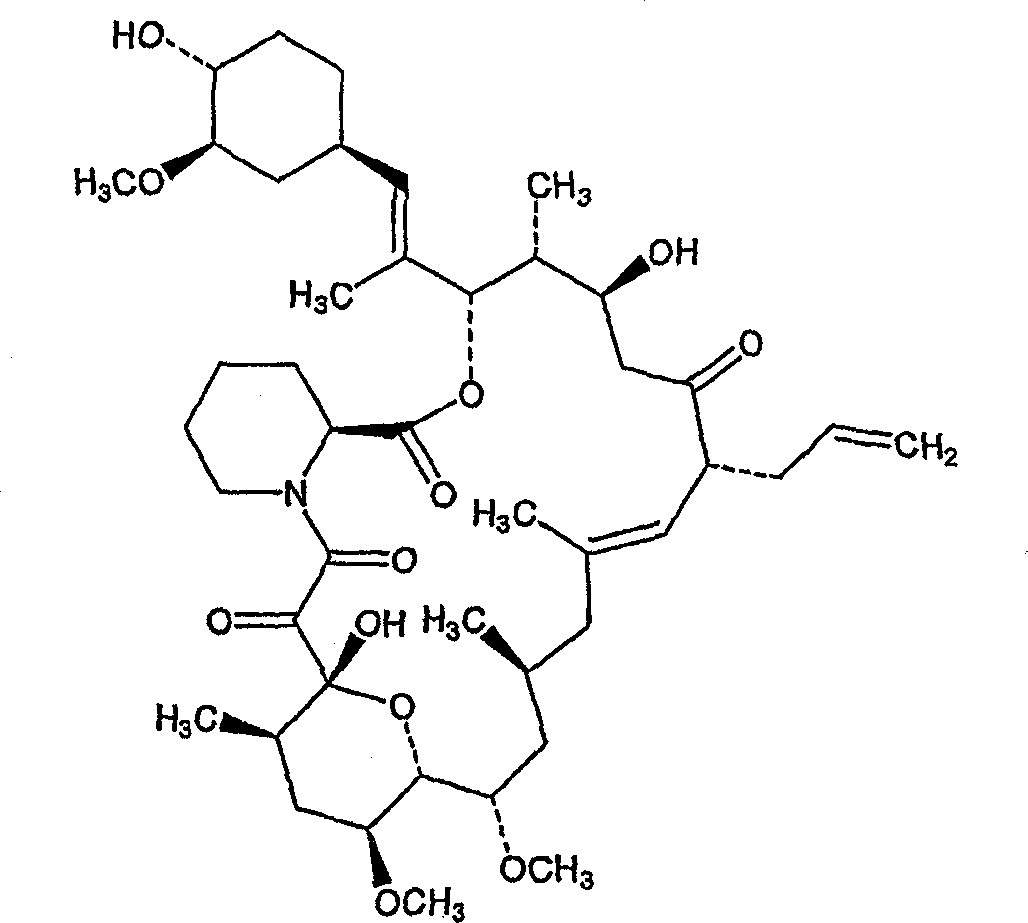
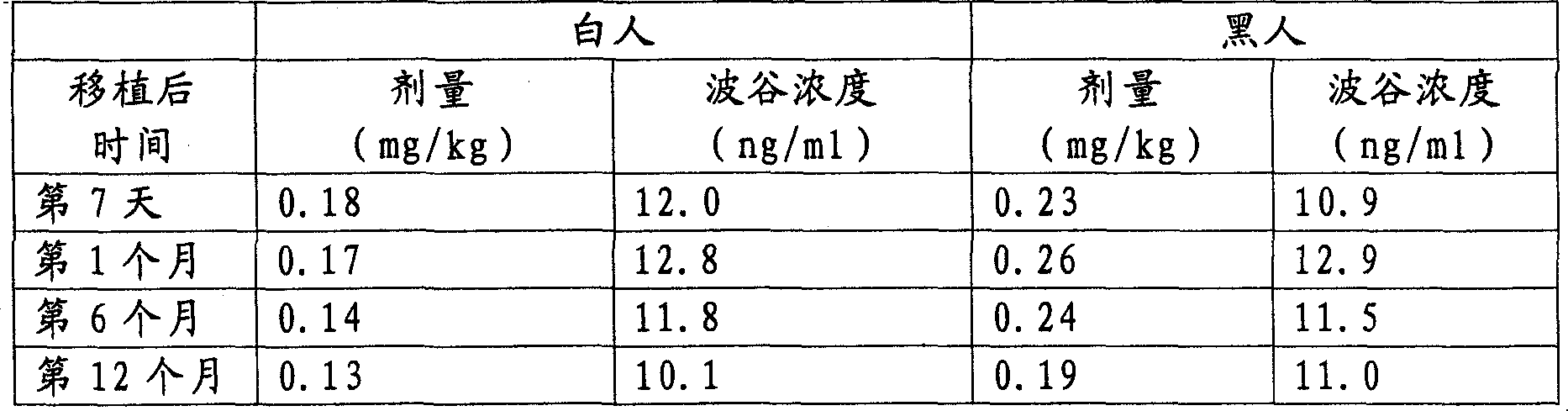
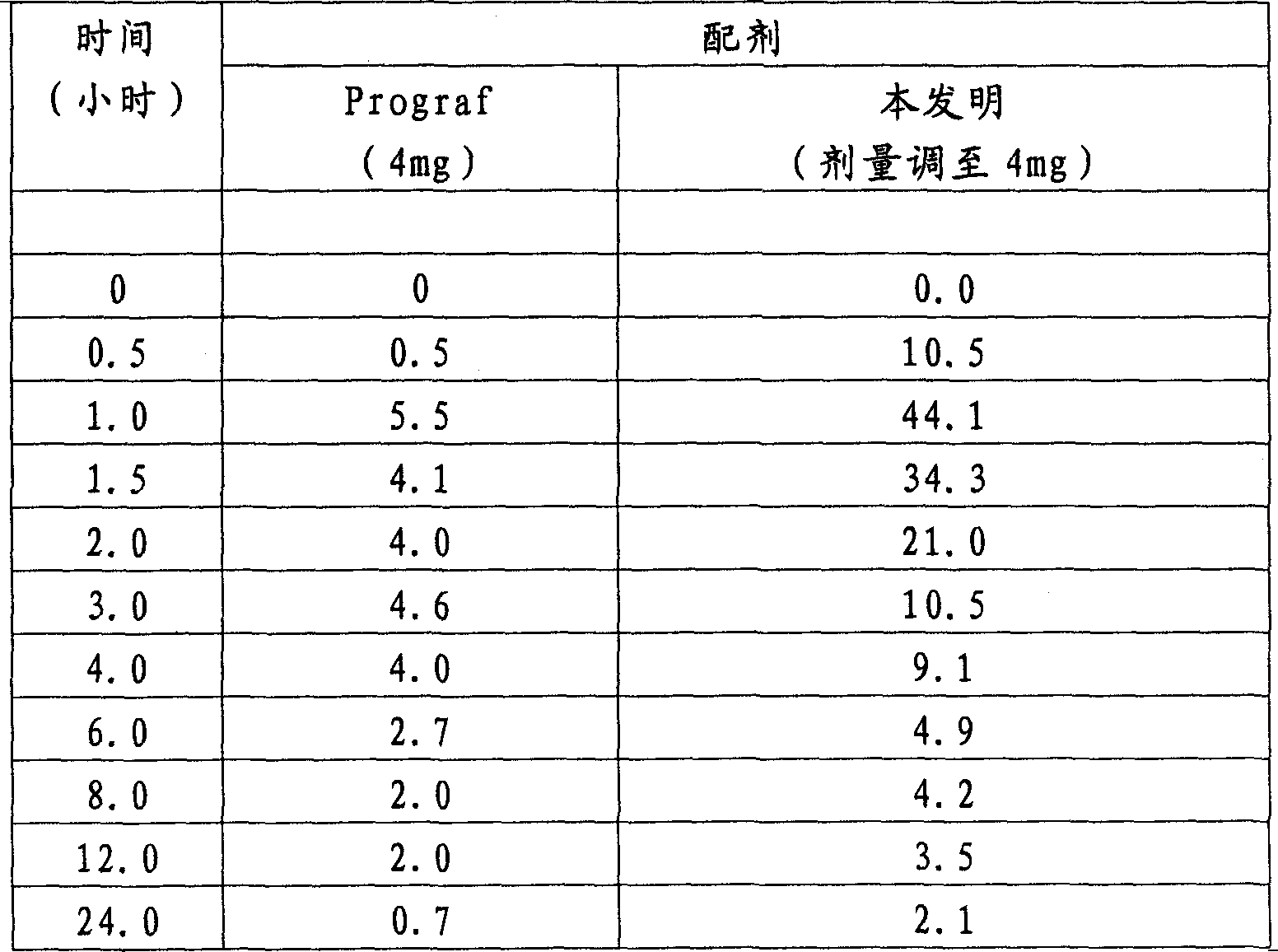
Solid dispersions comprising tacrolimus
A technology of active ingredients and compositions, applied in the field of solid dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0209] Example 1: Immediate release tablet with enhanced bioavailability
[0210] Tablet Composition:
[0211]
%
mg
Tacrolimus
0.50
1.00
Lactose 200 mesh
49.75
100.00
PEG 6000
34.48
69.30
Poloxamer 188
14.78
29.70
Magnesium stearate
0.50
1.01
total
100.00
201.01
[0212] Tacrolimus was dissolved in polyethylene glycol 6000 and poloxamer 188 (w / w ratio 70:30) at 70°C. The solution was sprayed onto 250 g of lactose in a fluidized bed Strea-1. The granulated product was sieved through a 0.7mm sieve and mixed with magnesium stearate in a Turbula mixer for 0.5 minutes. The mixture is compressed into 8 mm tablets (200 mg tablet, compound cup shape) containing 1 mg of active ingredient.
[0213] Average disintegration time: 20 minutes. Hardness: 45N.
Embodiment 2
[0214] Example 2: PEG 6000 / poloxamer 188 based immediate release tablet Tablet composition:
[0215] substance
%
mg
Tacrolimus
1.98
2.00
Lactose monohydrate, lactose 200 mesh
40.50
40.91
PEG 6000
33.26
33.60
Poloxamer 188, Lutrol 68
14.40
14.40
[0216] Magnesium stearate
0.50
0.51
talc
4.50
4.55
Croscarmellose Sodium, Ac-Di-Sol
5.00
5.05
100.00
101.01
[0217] Tacrolimus was dissolved in PEG 6000 at a temperature above 80°C. Poloxamer 188 was added and the solution was heated to a temperature above 80°C. The solution was sprayed onto 200 g of lactose monohydrate in a fluidized bed Phast FB100 using a dosing device Phast FS 1.7. The resulting granules were sieved with a Comill (sieve size 1397) at 4500 rpm and mixed with croscarmellose sodium in a Turbula mixer for 3 minutes.
[0218] The magnesium stearate...
Embodiment 3
[0223] Example 3: Enteric Coating of the Immediate Release Tablet of Example 2
[0224] The enteric coating is based on the acrylic polymer Eudragit L30D-55. Eudragit L 30D is supplied as an aqueous latex suspension which forms a water insoluble film when the water evaporates during the coating process. The polymer is insoluble at pH values below 5.0 and readily soluble at pH values above 6.0. Tablets prepared as described in Example 2 were coated with the following film coating composition:
[0225] substance
w / w%
Eudragit L30D-55
40
water
52
triethyl citrate
1.8
Antifoam Lotion
0.2
Talc (fine)
6
total
100
[0226] The amount of film polymer (Eudragit) applied was calculated on the basis of mg of film polymer per cm2 of tablet surface. The thickness of the enteric coating is 80 μm. Determination of the film thickness used was based on the measurement of the tablet height increm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com