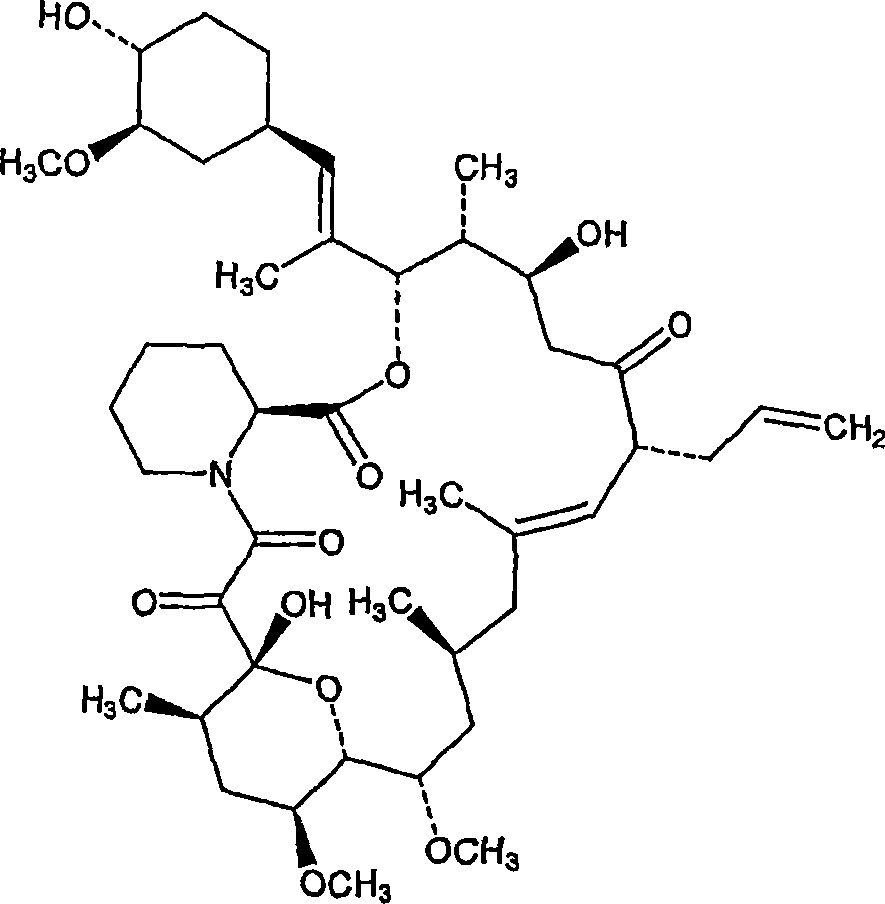
Solid dispersions comprising tacrolimus
A technology of solid dispersion and tacrolimus, applied in medical preparations containing active ingredients, organic active ingredients, liquid delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0207] Example 1: Immediate release tablet with enhanced bioavailability
[0208] %
mg
Tacrolimus
0.50
1.00
Lactose 200 mesh
49.75
100.00
PEG 6000
34.48
69.30
Poloxamer 188
14.78
29.70
0.50
1.01
total
100.00
201.01
[0209] Tacrolimus was dissolved in polyethylene glycol 6000 and poloxamer 188 (w / w ratio 70:30) at 70°C. The solution was sprayed onto 250 g of lactose in a fluidized bed Strea-1. The granulated product was sieved through a 0.7mm sieve and mixed with magnesium stearate in a Turbula mixer for 0.5 minutes. The mixture is compressed into 8 mm tablets (200 mg tablet, compound cup shape) containing 1 mg of active ingredient.
[0210] Average disintegration time: 20 minutes. Hardness: 45N.
Embodiment 2
[0211] Example 2: PEG 6000 / poloxamer 188 based immediate release tablet
[0212] substance
%
mg
Tacrolimus
1.98
2.00
Lactose monohydrate, lactose 200 mesh
40.50
40.91
PEG 6000
33.26
33.60
Poloxamer 188, Lutrol 68
14.40
14.40
0.50
0.51
4.50
4.55
Croscarmellose Sodium, Ac-Di-Sol
5.00
5.05
100.00
101.01
[0213] Tacrolimus was dissolved in PEG 6000 at a temperature above 80°C. Poloxamer 188 was added and the solution was heated to a temperature above 80°C. The solution was sprayed onto 200 g of lactose monohydrate in a fluidized bed Phast FB100 using a dosing unit Phast FS1.7. The resulting granules were sieved with a Comill (sieve size 1397) at 4500 rpm and mixed with croscarmellose sodium in a Turbula mixer for 3 minutes.
Embodiment 3
[0218] Example 3: Enteric Coating of the Immediate Release Tablet of Example 2
[0219] substance
[0220] The amount of film polymer (Eudragit) applied is based on each cm 2 Calculated in mg of film polymer on the tablet surface. The thickness of the enteric coating is 80 μm. Determination of the film thickness used was based on the measurement of the tablet height increments with a digital micrometer. The film coating process was carried out in a Phast FB100 fluidized bed equipped with a Wurster-like insert, inlet air temperature 50°C, inlet airflow 100 cbm / hour, product temperature 38°C, feed rate 15 g / min.
[0221] The coated tablets were cured in an oven at 30°C for 48 hours. Alternatively, the coated tablet can be more efficiently cured at 40°C for 24 hours.
[0222] Enteric-coated tablets were subjected to in vitro dissolution testing using two different dissolution media / test methods.
[0223] time (hours)
[0224] time (minutes)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com