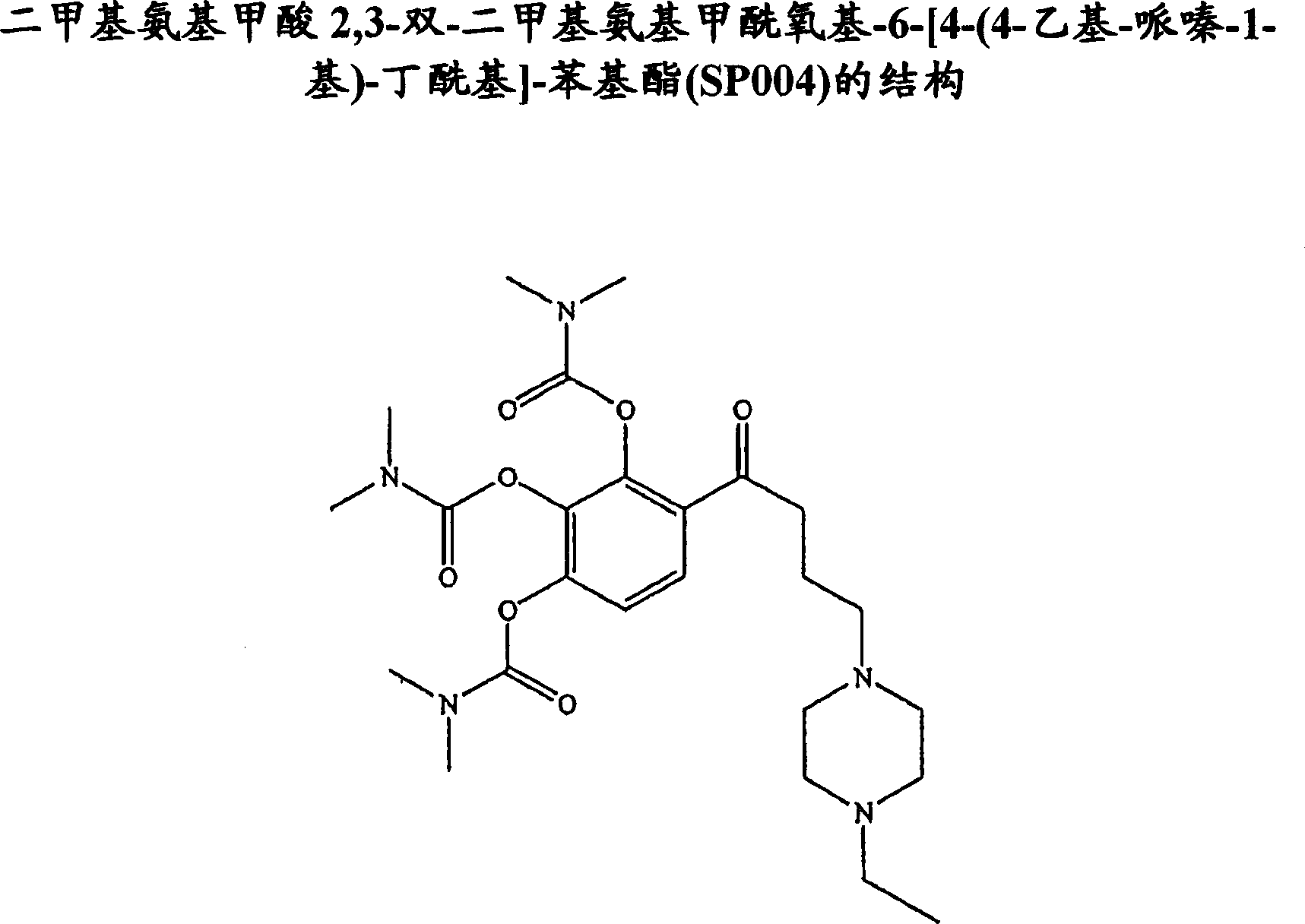
Sigma-1 receptor ligand with acetylcholinesterase
A carrier, an effective amount of technology, applied in the field of prevention or treatment of neurodegenerative diseases, can solve problems such as failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
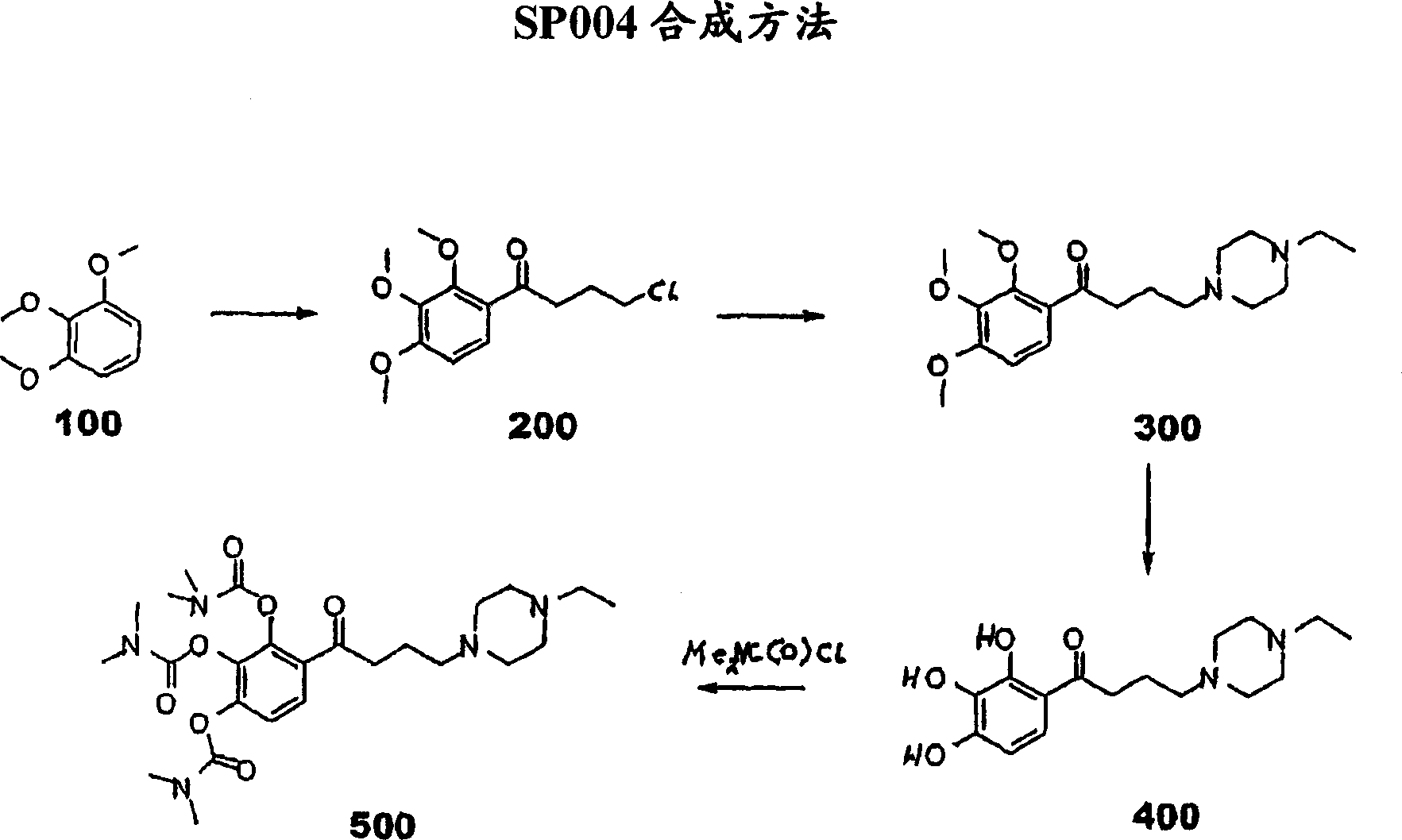
[0071] The synthesis of embodiment 1.SP004
[0072] Such as figure 2 As shown, 10 grams (0.059mol) of 2,3,4-trimethoxy-benzene ( figure 2 "100" in ) was added to a suspension of aluminum chloride (35.5 g, 0.26 mol) in carbon disulfide. Keeping the temperature at about 10°C, gamma-chlorobutyryl chloride (14.7 g, 0.1 mol) was added. After the addition was complete, stirring was continued at room temperature for two hours. The reaction mixture was poured into ice and extracted with dichloromethane. The organic layer was separated, washed with water, MgSO 4 dry. The solution was concentrated under reduced pressure. The residue was used in the next step without further purification.
[0073] In the next step, the compound 4-chloro-1-(2,3,4-trimethoxy-phenyl)-butan-1-one ( figure 2 "200") (7 g, 0.026 mol) and N-ethylpiperazine (5.8 g, 0.051 mol) for seven hours. After distilling off unreacted N-ethylpiperazine, the residue was chromatographed on silica gel.
[0074] In the...
Embodiment 2
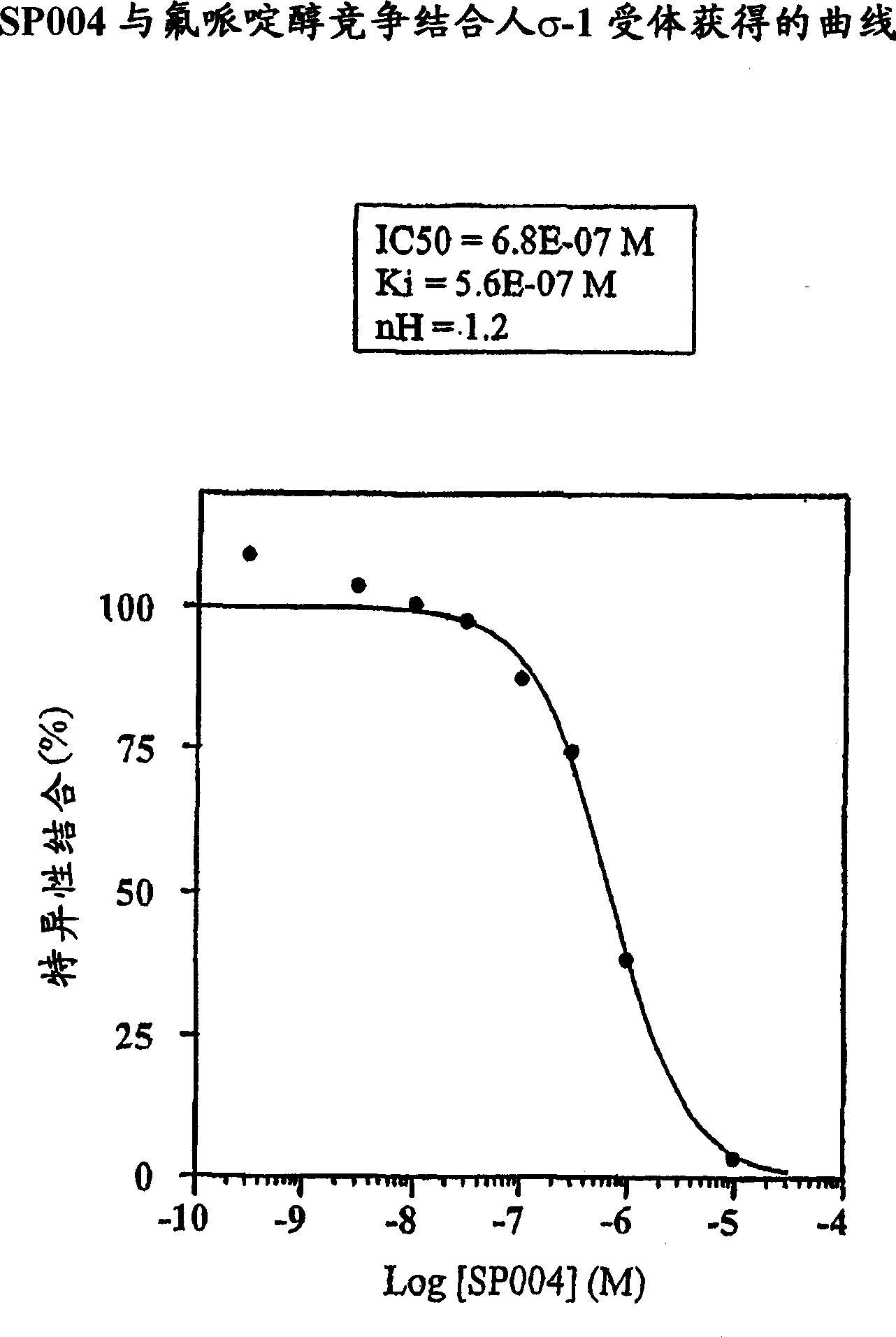
[0077] SP004 Binding Assay
[0078] Different binding studies were performed with the following SP004 concentrations:
[0079] 3E-10, 3E-9, 1E-8, 3E-8, 1E-7, 3E-7, 1E-6, 1E-5M.
[0080] Materials and methods
[0081] Central imidazoline-2 receptor (I 2 ). The test uses central I extracted from rat cortex 2 receptor. Each increasing concentration of SP004 and 2nM specificity I 2 receptor ligand [ 3 H]-Imidazole was incubated at 22°C for 30 minutes. Brown et al., Brit. J. Pharmacol., 99:803-809 (1990).
[0082] Muscarinic receptors (nonspecific). This test uses muscarinic receptors extracted from rat cortex. Each increasing concentration of SP004 and 0.05nM muscarinic ligand [ 3 H]-QNB was incubated at 22°C for 120 minutes. Richards, Brit. J. Pharmacol., 99:753-761 (1990).
[0083] Neuronal nicotinic α-BGTX-insensitive receptors. The assay uses neuronal nicotinic α-BGTX-insensitive receptors extracted from rat cortex. Each increasing concentration of SP004 and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com