New acyclic nucleoside phosphate ester and its pharmaceutical use
A pharmaceutical and drug technology, applied in the field of acyclic nucleoside phosphonate derivatives, can solve the problems of toxicity and kidney production, and achieve strong anti-hepatitis B virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
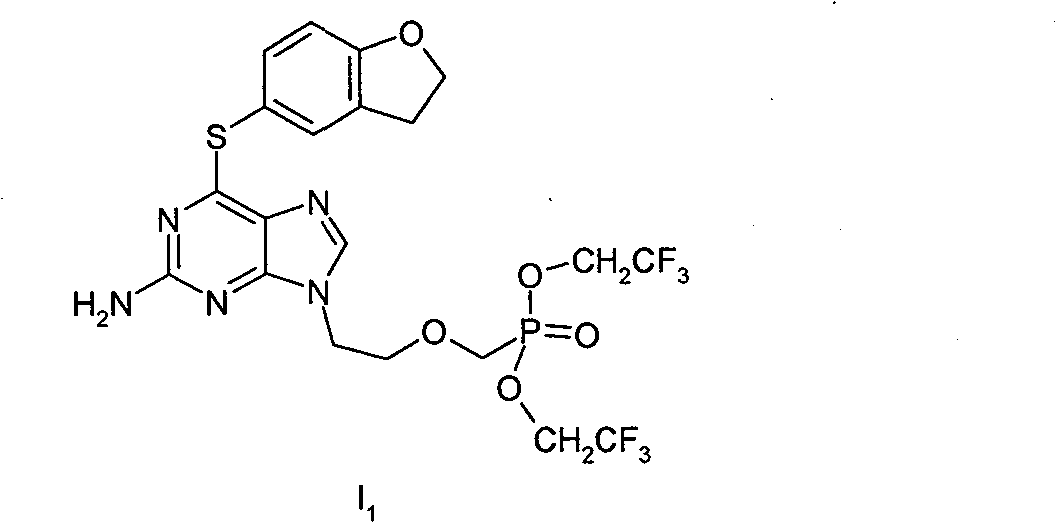
[0027] Example 1 2-amino-6-[(2,3-dihydrobenzofuran-5-yl)-thio]-9-[2-[bis(2,2,2-trifluoroethyl)phosphine Acylmethoxy]ethyl]-purine (I 1 )
[0028] 1.1 2-[bis(2,2,2-trifluoroethyl)-phosphonomethoxy]-ethyl chloride (III 1 )
[0029] Under stirring, 32 g of phosphorus trichloride was slowly added to 70 g of trifluoroethanol, and stirred at 85°C for 5 hours. Fractional distillation under reduced pressure collected the components at 120-125°C / 70-74 mmHg to obtain 62 grams of tris-(2,2,2-trifluoroethyl)phosphite.
[0030] Add 39 grams of 2-chloroethanol and 15 grams of paraformaldehyde to 80 ml of dichloromethane, cool in an ice bath; pass hydrogen chloride under stirring for 10 hours, and continue stirring overnight. Then the water layer was separated, the organic layer was dried with anhydrous calcium chloride, the solid was filtered off, the solvent was evaporated under reduced pressure, fractional distillation was carried out, and the components at 75-78°C / 24-26 mmHg were col...
Embodiment 2
[0038] Example 2 2-amino-6-[(benzo-1,3-dioxol-5-yl)-thio]-9-[2-[bis(2,2,2-trifluoroethane) base) phosphonomethoxy] ethyl] - purine (I 2 )
[0039] 2.1 (Benzo-1,3-dioxol-5-yl)-thiol (V 2 )
[0040] Add 2.54 milliliters of bromine dropwise to a solution of 4 grams of benzo-1,3-dioxolene in 44 milliliters of glacial acetic acid. After the addition, continue stirring for 2 hours, evaporate the reaction solution to dryness under pressure, and add ice water , extracted with ether, washed with aqueous sodium bicarbonate solution, dried with anhydrous sodium sulfate, and evaporated to dryness under pressure, the residue was fractionally distilled, and the fraction at 73°C / 0.1mmHg was collected to obtain 4.2 g of a colorless liquid.
[0041] Suspend 0.61 g of magnesium chips in 10 ml of anhydrous tetrahydrofuran, and add dropwise a solution of 4.2 g of 5-bromo-benzo-1,3-dioxolene in 10 ml of tetrahydrofuran under nitrogen within 1 hour. After the dropwise addition was completed, th...
Embodiment 3
[0045] Example 3 2-amino-6-[(benzo-1,4-dioxen-6-yl)-thio]-9-[2-[bis(2,2,2-trifluoroethane base) phosphonomethoxy] ethyl] - purine (I 3 )
[0046] 3.1 (Benzo-1,4-dioxan-6-yl)-thiol (V 3 )
[0047] Referring to Method 2.1, benzo-1,4-dioxene was reacted with bromine to obtain 6-bromo-benzo-1,4-dioxene; the latter was reacted with magnesium chips and sulfur to obtain V 3 .
[0048] 3.2 2-amino-6-[(benzo-1,4-dioxen-6-yl)-thio]-9-[2-[bis(2,2,2-trifluoroethyl) Phosphonomethoxy] ethyl] - purine (I 3 )synthesis
[0049] Refer to Method 1.4, V 3 with IV 1 condensation to produce I 3 . 1 HNMRδ (ppm, CDCl 3 ): 7.42(s, 1H); 6.96(d, 1H); 6.90(dd, 1H); 6.80(d, 1H); 4.75(b, 2H); 4.43(m, 4H); 4.25(m, 4H) ; 3.94(m, 2H); 3.72(t, 2H); 3.0(t, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com