Pressurized metered dose inhalers and pharmaceutical aerosol formulations
a technology of aerosol formulation and metered dose, which is applied in the directions of aerosol delivery, dispersion delivery, organic active ingredients, etc., can solve the problems of difficult formulation of conventional aerosols, and short shelf life of formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 4-7
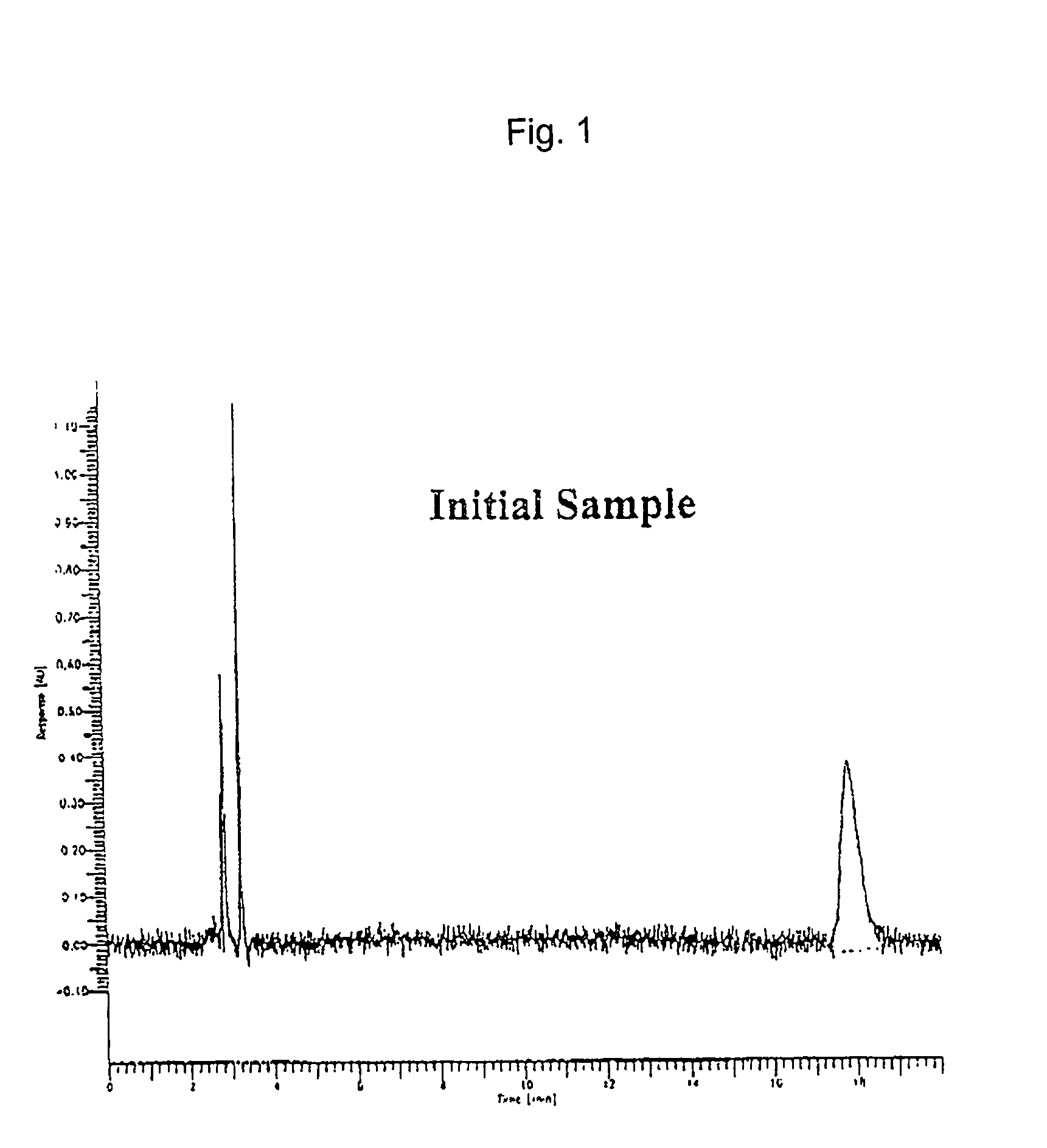
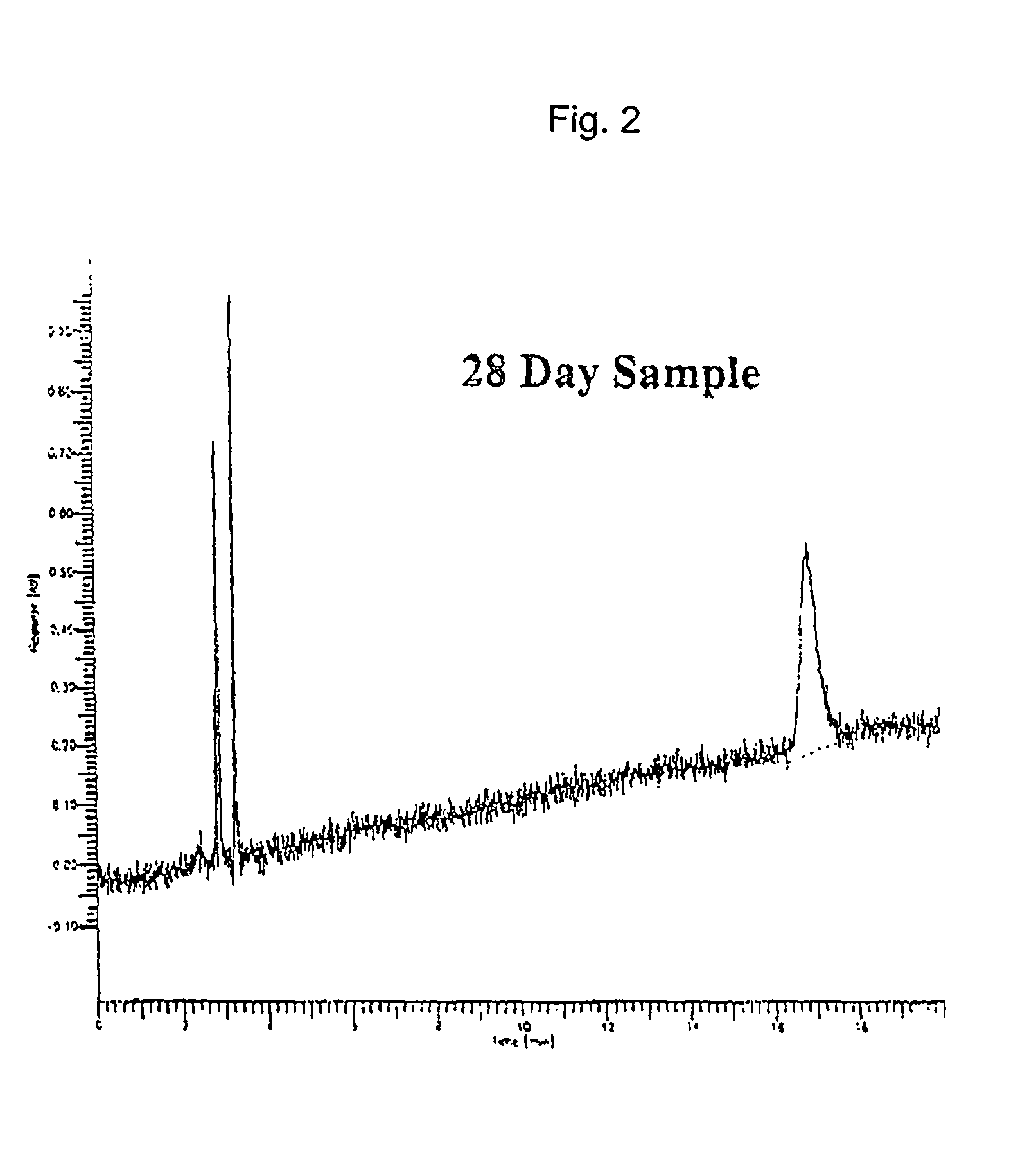
[0058] Four solution aerosols according to present invention were formulated by combining the components shown in Table 2, using the method described in Example 1. To determine the stability of the solution aerosol formulations, Examples 6 and 7 were maintained for 1 month (28 days) at 40.degree. C. and 75% relative humidity, which are considered herein as accelerated conditions. The solution aerosol formulations were equilibrated at room temperature overnight before testing. The properties of the solution aerosol formulations were measured as in Example 1 and the results are shown in Table 2.
[0059] The data indicates that the dose delivered (by unit spray determination) after storage under accelerated conditions was lower than that obtained with the initial samples due to drug adsorption onto the valve gasket material. However, the solution aerosol formulations showed no signs of chemical deterioration.
examples 8 and 9
[0060] Two solution aerosols according to present invention were formulated by combining the components shown in Table 3, using the method described in Example 1. To determine the stability of the solution aerosol formulations, Example 9 was maintained for 1 month (28 days) at 40.degree. C. and 75% relative humidity, which are considered herein as accelerated conditions. The solution aerosol formulations were equilibrated at room temperature overnight before testing. The properties of the solution aerosol formulations were measured as in Example 1 and the results are shown in Table 3.
[0061] The drug could not be recovered from the gasket materials during this study, which resulted in a loss of about 15% by weight. However, the solution aerosol formulations showed no signs of chemical deterioration.
examples 10-13
[0062] Four suspension aerosols according to present invention were formulated by combining the components shown in Table 4, using the method described in Example 1. To determine the stability of the suspension aerosol formulations, Examples 12 and 13 were maintained for 1 month (28 days) at 40.degree. C. and 75% relative humidity, which are considered herein as accelerated conditions. The suspension aerosol formulations were equilibrated at room temperature overnight before testing. The properties of the suspension aerosol formulations were measured as in Example 1 and the results are shown in Table 4.
[0063] After 28 days storage, the dose delivered (by unit spray determination) in Examples 12 and 13 was less than that obtained with the initial Examples 10 and 11, but not reduced by the same degree as with the solution formulation examples.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com