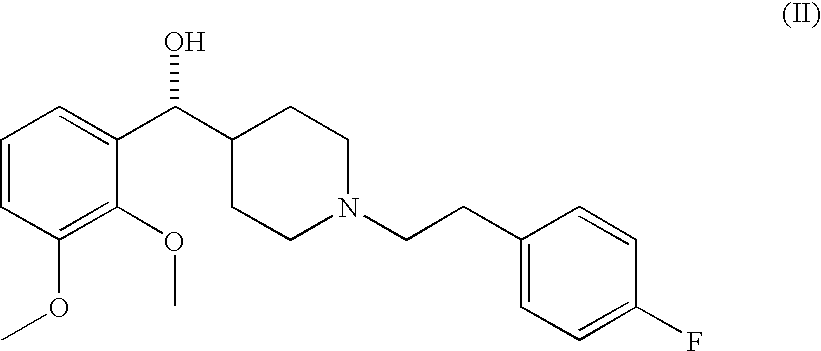
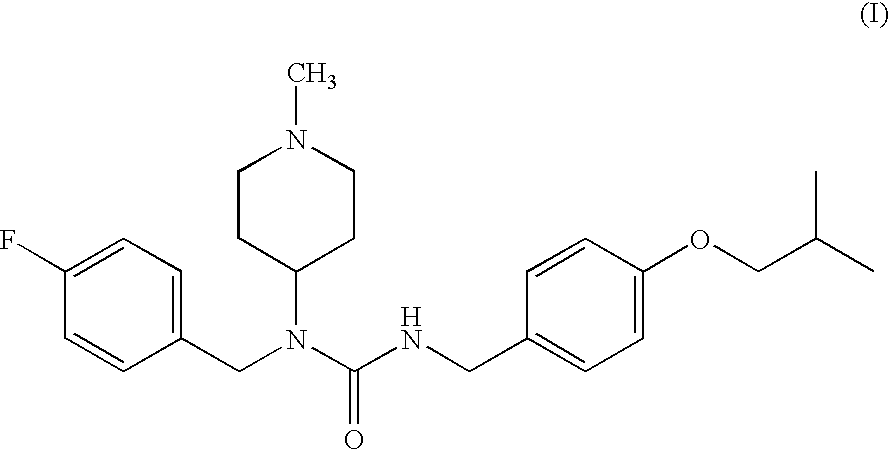
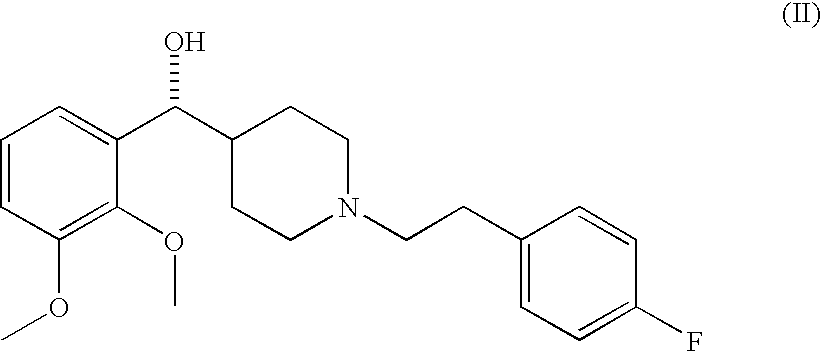
Selective serotonin receptor inverse agonists as therapeutics for disease
a serotonin receptor and inverse agonist technology, applied in the field of disease therapy with selective serotonin receptor inverse agonists, can solve the problems of reducing the societal productivity of individuals affected by this disease, reducing the use of heterocyclic compounds, and reducing the use of drugs. the effect of reducing the risk of adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Definitions
[0020] For the purpose of the current disclosure, the following definitions shall in their entireties be used to define technical terms, and shall also, in their entireties, be used to define the scope of the composition of matter for which protection is sought in the claims.
[0021]“Constitutive activity” is defined as the elevated basal activity of a receptor that is independent of the presence of an agonist. Constitutive activity of a receptor may be measured using a number of different methods, including cellular (e.g., membrane) preparations (see, e.g., Barr &. Manning, J. Biol. Chem. 272:32979-87 (1997)), purified reconstituted receptors with, or without the associated G-protein in phospholipid vesicles (Cerione et al., Biochemistry 23:4519-25 (1984)), and functional cellular assays (U.S. Patent Application Ser. No. 60 / 103,317) or any other method known in the art.
[0022]“Agonist” is defined as a compound that increases the basal activity of a receptor when it cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmaceutical composition | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| blood pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com