Compositions and methods of use comprising substances with neural plasticity actions administered at non-psychedelic/psychotomimetic dosages and formulations
A compound, dimethyl technology, used in pharmaceutical combinations, chemical instruments and methods, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
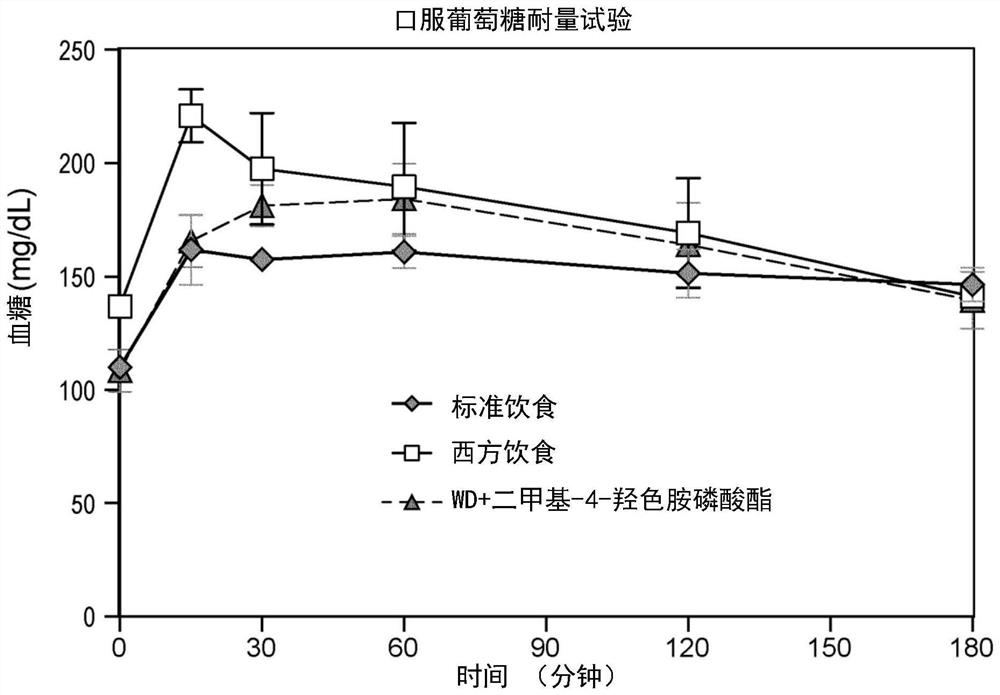
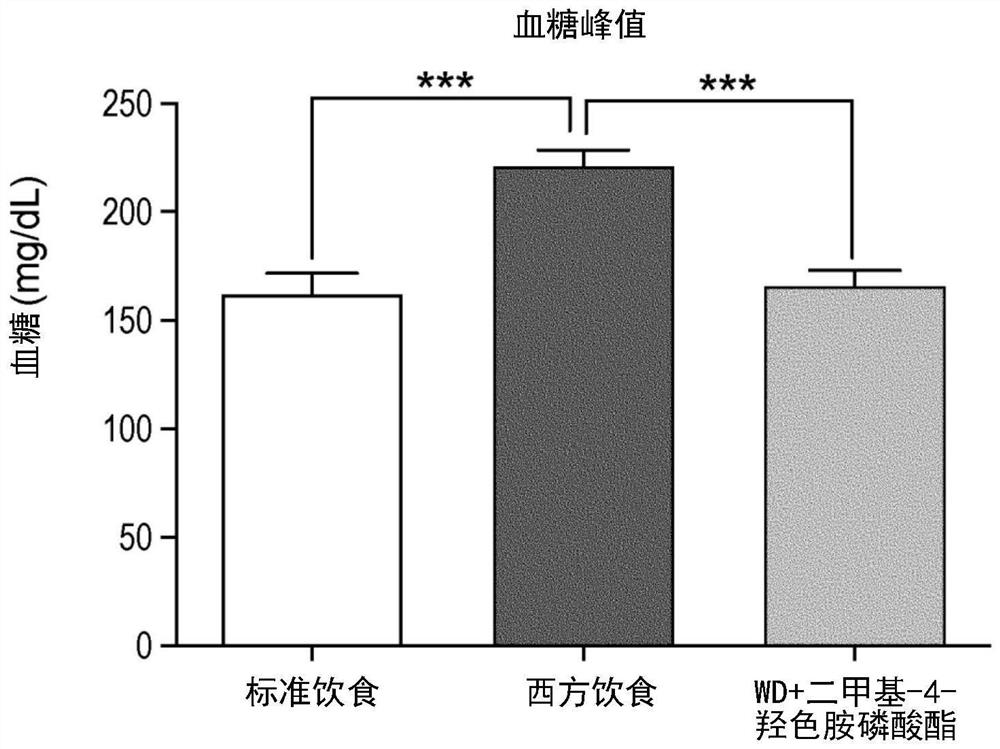
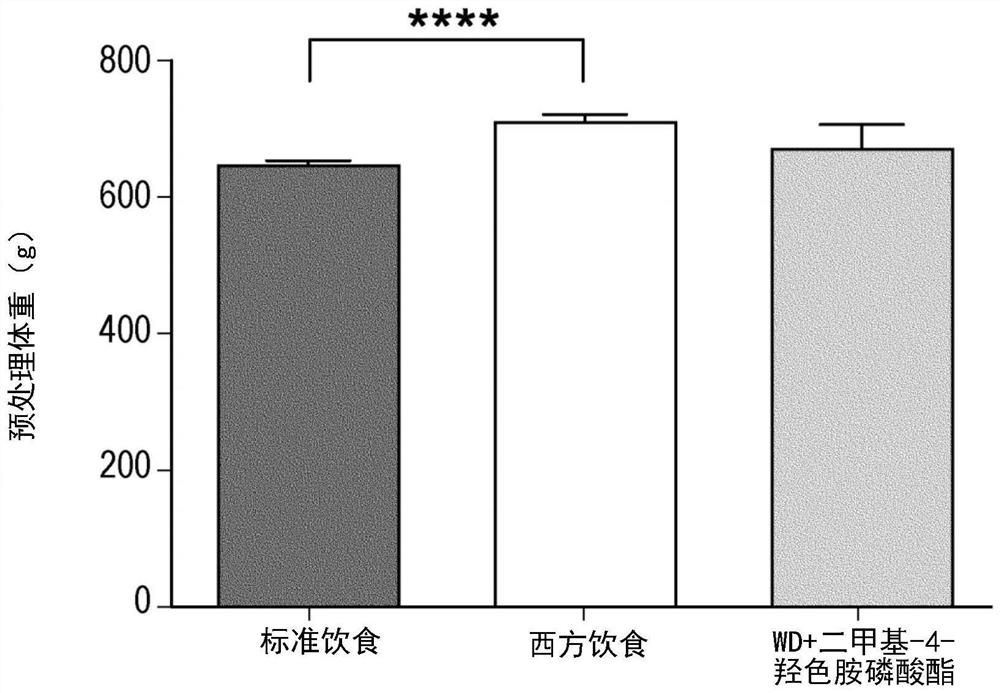
[0270] The following clinical observations were made in the Netherlands on individuals self-administering sclerotia of Psilocibeatlantis (magictruffles.com). Psilocybeatlantis is a species containing dimethyl-4-hydroxytryptamine phosphate, dimethyl-4-hydroxytryptamine, monomethyl psilocybin and ω-N-methyl-4-hydroxytryptamine (Guzmán G, Hanlin RT, White C. Another new bluing species of Psilocybe from Georgia, U.S.A. Mycotaxon 2003; 86:179–183).
[0271] It is worth noting that the sale and use of sclerotia-like psychedelic mushrooms is legal in the Netherlands, and the manufacturer of the particular brand and species of sclerotia self-administered by the individual stated that the "psychedelic dose" ingested 15 grams [For the first individual, the intake of CF (1.5 grams) was 1 / 10 of the recommended "psychedelic dose", and the intake of the other two individuals GG and PM (3 grams) was the recommended "psychedelic dose". 1 / 5 of the phantom dose].
[0272] The inventors descri...
Embodiment 2
[0303] Example 2 - In vivo studies
[0304] To date, the scientific literature available in clinical settings (safety and efficacy studies) and experimental settings (in vitro and in vivo experimental settings, including the work of Ly et al., 2018) still focuses on Pulse therapy, expected and intended to produce clinically meaningful psychedelic / hallucinogenic effects (Johnson M1, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008 Aug;22(6):603-20). A similar approach (pulse exposure to high concentrations) was used in an experimental trial (Ly et al., 2018) that supports the use of psychedelic doses of 5-HT2A agonists.
[0305] To assess whether repeated chronic low-dose (non-psychedelic / hallucinogenic) administration of 5-HT2A agonists potentially modulates neuroplasticity and / or modulates neuroinflammation, and to assess whether these effects are potentially cytoprotective, and these Whether the effect may lead to a pote...
Embodiment 3
[0358] Example 3 - In vitro studies
[0359] To assess the mechanism of potential therapeutic effects and whether repeated chronic low-dose treatment with 5-HT2A agonists might modulate neuroplasticity and / or modulate neuroinflammation, and to assess whether these effects are potentially cytoprotective, including against excitotoxicity The inventors performed a series of preclinical in vitro experiments targeting inflammatory mediators and, ultimately, whether these effects would lead to potentially clinically meaningful therapeutic effects. These tests were specifically designed to assess the potential therapeutic effect of repeated neuroplastic doses (low concentrations) classified as 5-HT2A agonists.
[0360] 1. Effects of dimethyl-4-hydroxytryptamine and dimethyl-4-hydroxytryptamine carbamate on NMDAR subunits in ARPE19 cells and 5-HT2 receptor subtypes
[0361] suppose :
[0362] The membranes of retinal pigment cells (ARPE-19 cell line) express NMDARs and 5-HT2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com