Phylloline derivatives and their pharmaceutical compositions and their application in pharmacy
A technology of anophylline and its derivatives, which is applied in the field of anophylline derivatives 1-2 and their pharmaceutical compositions, and can solve problems such as those that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
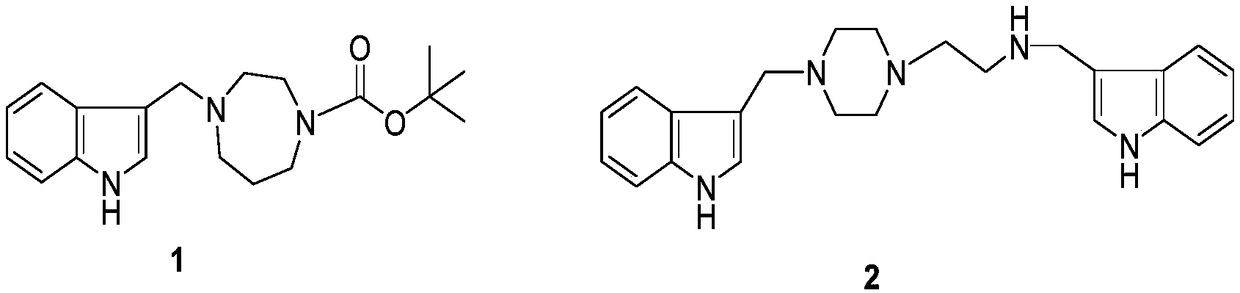
[0025] Phylloline (2mmol) and N-tert-butoxycarbonyl-homopiperazine (2mmol) were dissolved in 10 mL of anhydrous toluene, refluxed at 110°C until the end of the reaction, and the solvent was recovered under reduced pressure to prepare a crude product, which was prepared by silica gel column layer Purify by analysis (diethylamine / methanol / chloroform=2 / 4 / 94) to obtain the target compound (1).
[0026] Compound 1 structure determination data:
[0027] N-tert-Butyloxycarbonyl-N-homopiperazinyl-3-indolylmethylamine (N-tert-Butyloxycarbonyl-N-homopiperazinyl-3-indolymethylamine,
[0028]
[0029] 1.85-1.82(m,2H,H-3'),1.49(s,9H,Me-Boc). 13 C NMR (100MHz, CDCl 3 )δ:155.7(s,C-8),136.4(s,C-8),127.9(s,C-9),123.8(d,C-2), 121.8(d,C-6),119.5( d,C-5),119.3(d,C-4),112.8(s,C-3),111.2(d,C-7),79.4(s,C-Boc),55.77(t,C-7 '),54.7(t,C-2'),53.5(t,CHN),46.8(t,C-6'),46.2(t,C-4'),28.6(s,C-Me-Boc) ,27.9(t,C-3').ESIMS:m / z 330 [M+H] + ,HRESIMS:C 19 h 27 N 3 o 2 [M+H] + The measured value is 330...
preparation Embodiment 2
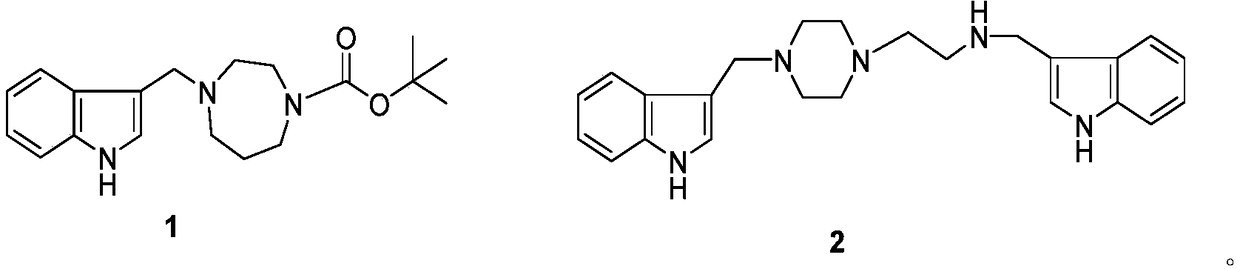
[0031] Phylloline (4mmol), dissolved in 10mL of anhydrous toluene with 2-aminoethylpiperazine (2mmol), refluxed at 110°C until the end of the reaction, recovered the solvent under reduced pressure, prepared a crude product, and performed silica gel column chromatography ( Diethylamine / methanol / chloroform=3 / 5 / 92) to obtain the target compound (2).
[0032] Compound 2 structure determination data:
[0033] N-(N-3-indolylmethylaminoethyl)-N-piperazinyl-3-indolylmethylamine [N-[N-(3-indolymethyl)-aminoethyl]-N-Piperazinyl-3-indolylmethyla
[0034]
[0035] mine,2]: white powder, yield 70%. 1 H NMR (CDCl 3 ,400MH Z )δ H:8.72(s, 2H,NH),7.72-7.01(m,10H,H-2,2',4,4',5,5',6,6',7,7'),3.98(s, 2H, CH 2 N),3.72(s,2H,CH 2 CH 2 NH CH 2 ),3.45(s,1H,CH 2 CH 2 NH CH 2 ), 2.79-2.77(m,2H,CH 2 CH 2 NHCH 2 ),2.69-2.68(m,2H, CH 2 CH 2 NHCH 2 ), 2.52-2.42(m,4H,H-3',5'),2.20-2.18(m,4H,H-2',6'). 13 C NMR (100 MHz, CDCl 3 )δ:136.4(s,C-8,8'),128.1(s,C-9),127.0(s,C-9'),124.1(...
preparation Embodiment 1
[0051] The anophylline derivatives 1-2 prepared according to the method of Preparation Example 1 were dissolved in a small amount of DMSO separately or in combination, and then water for injection was added as usual, finely filtered, potted and sterilized to make an injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com