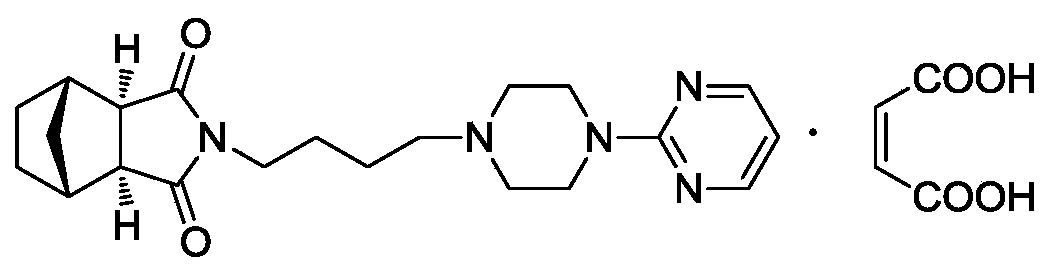
A serotonin receptor agonist
A solvent-to-volume ratio technology, applied in the field of maleate of tandospirenone, can solve problems such as differences in drug quality and clinical efficacy, affecting the stability of drug preparations, solubility, hygroscopicity, and bioavailability, and achieve water solubility. Good properties, improved safety and efficacy, and increased bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 tandospirone maleate
[0044] Weigh 1 kg of tandospirone and 303 g of maleic acid, add 6L of ethanol, heat to solvent reflux, stir until the reaction is complete, stop heating, naturally cool to room temperature, filter and collect the solid, and dry to obtain 1.30 kg of tandospirone maleate Duspirone, the yield is 99.8%, mass spectrometry shows its ESI m / z: 383 (M + ).
Embodiment 2
[0045] The preparation of embodiment 2 tandospirone maleate
[0046] Tandospirone maleate was prepared according to the method of Example 1, see Table 1 for specific conditions, and the feeding amount of tandospirone was 1 kg. There is no significant difference between the structural analysis results of the product obtained under each condition and the structural analysis results of Example 1.
[0047] Table 1, the preparation condition of tandospirone maleate
[0048]
[0049]
[0050] The ratios of mixed solvents in the table are volume ratios.
Embodiment 3
[0051] Example 3 Preparation of Tandospirone Maleate Form II
[0052] Take 200g of tandospirone maleate, add 900mL of acetonitrile and 300mL of water, heat to solvent reflux, stir until completely dissolved, stop heating, naturally cool to room temperature for crystallization, collect crystals by filtration, and dry to obtain 193g of white powdered horseradish. The yield of tandospirone crystalline form II was 96.5%, and its ESI m / z was shown by mass spectrometry: 383 (M + ), the measured melting point is 159.0-160.5°C.
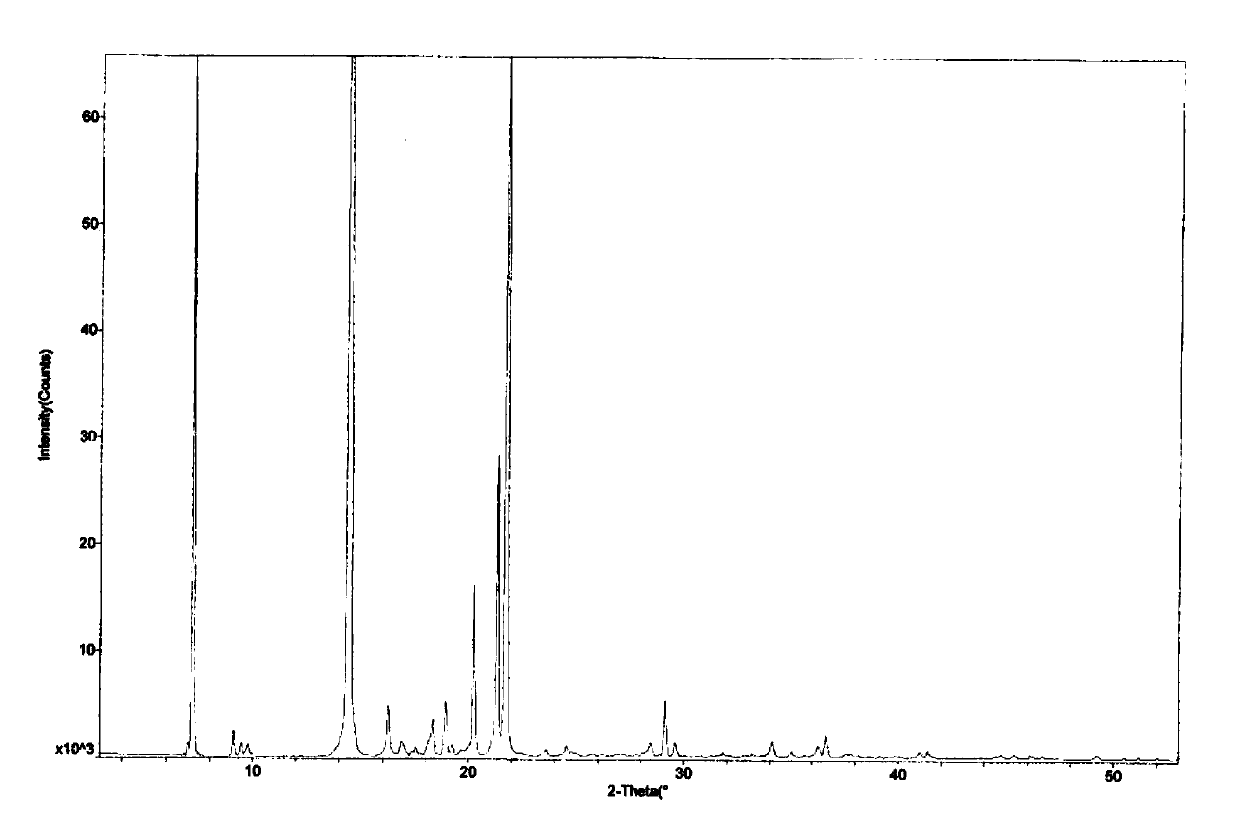
[0053] Adopt Cu Kα radiation, measure the X-ray powder diffraction result of tandospirone maleate crystal form II of the present invention as follows figure 1 The relevant diffraction data are shown in Table 2.
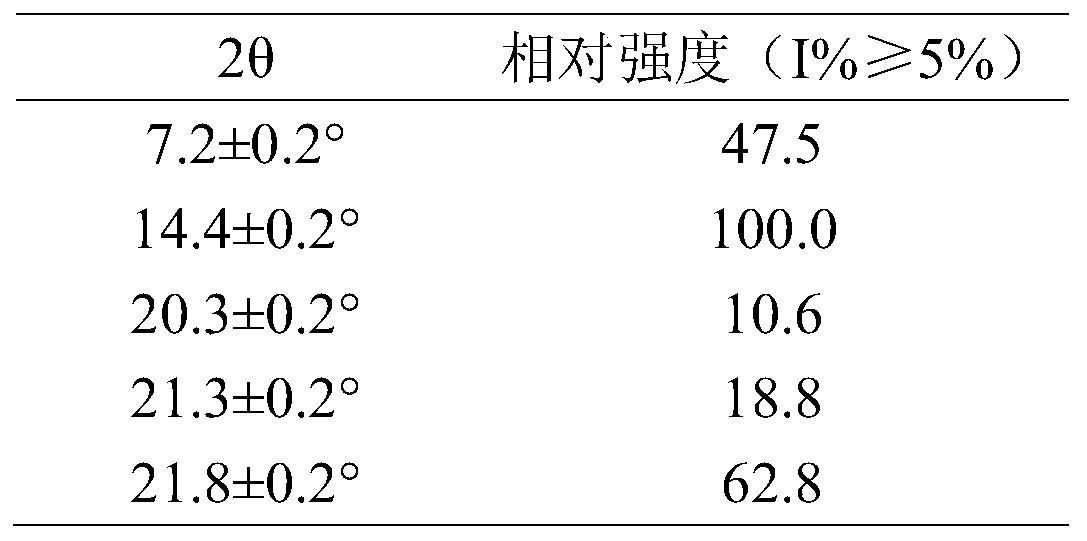
[0054] Table 2. X-powder diffraction results of crystal form II
[0055]
[0056]
[0057] Among them, the main relevant diffraction data of relative intensity I%≥5% are shown in Table 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com