Method of stimulating the motility of the gastrointestinal system using ipamorelin
A technology for gastrointestinal diseases and intestinal obstruction, which is applied in the field of stimulating gastrointestinal system motility, and can solve problems such as unacceptable pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
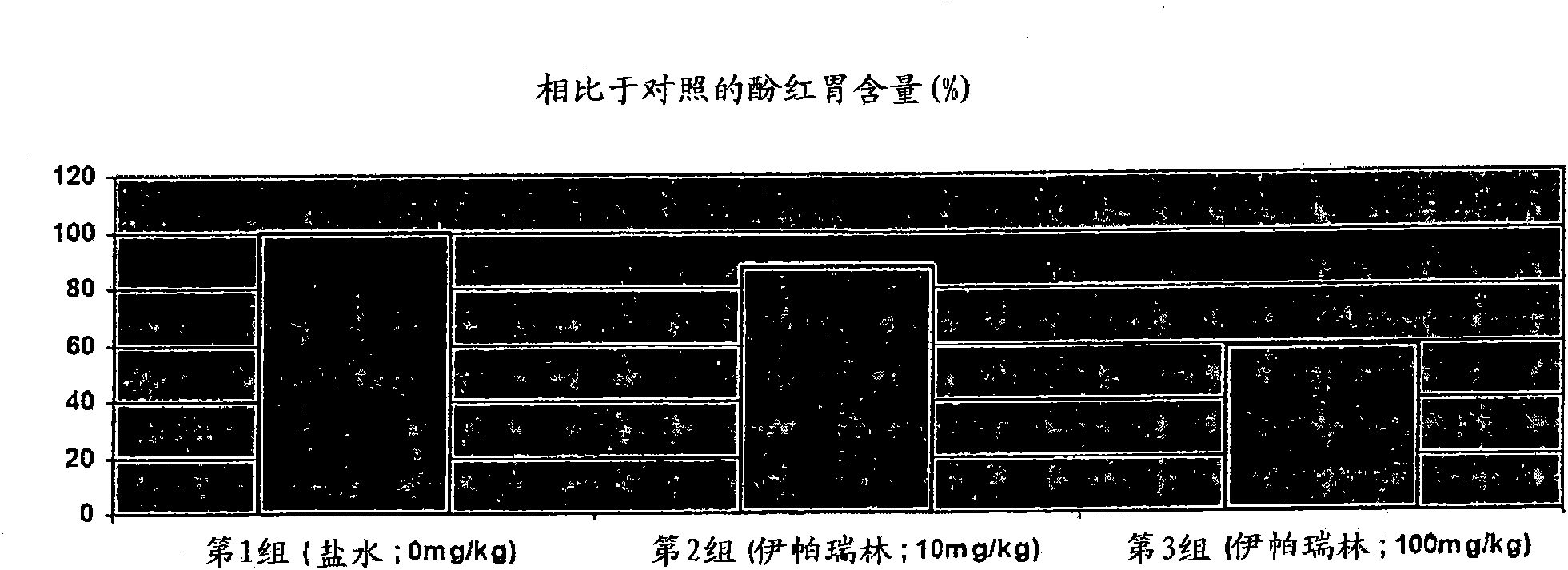
[0163] Example 1. Gastrokinetic efficacy of ipamorelin (10 or 100 mg / kg) in a rat model of postoperative ileus
[0164] This study evaluated the potential gastrokinetic efficacy of ipamorelin after a single oral dose of 10 or 100 mg / kg in a rat model of postoperative ileus.
[0165]
[0166] *This group of animals underwent all surgical procedures but no cecal manipulation.
[0167] Methods and Experimental Design
[0168] Male Sprague-Dawley obtained from Charles River Canada Inc. St. Constant, Quebec, Canada (Crl: (SD)) Rat (Rattus norvegicus). Animals were allowed to acclimatize to the laboratory environment for nine days between acquisition and start of handling. Animals were approximately 7 weeks old and weighed 230-254 g when treatment was initiated.
[0169] Animals were housed individually in stainless steel mesh bottom cages equipped with automatic water supply valves. Each cage is clearly marked with a color-coded cage card identifying project, group, an...
Embodiment 2
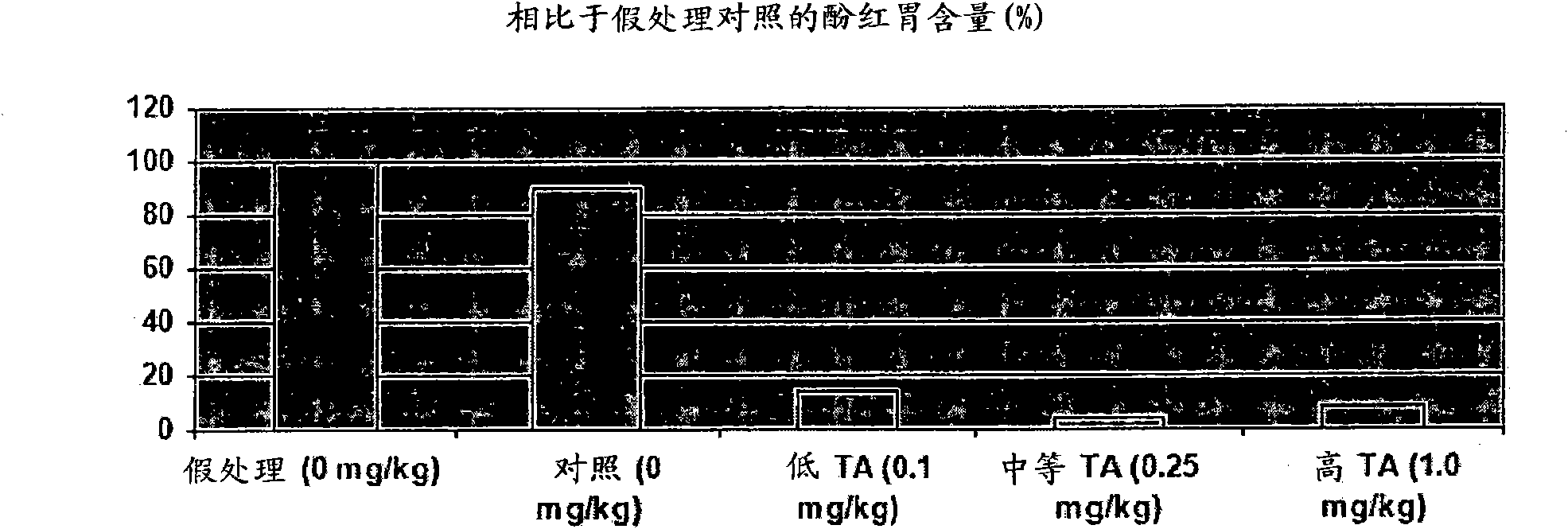
[0188] Example 2. Gastric Kinetic Efficacy of Intravenous Administration of Ipamorelin (0.1, 0.25 or 1.0 mg / kg) in a Rat Model of Postoperative Ileus
[0189] In this study, ipamorelin was administered as a slow bolus intravenous injection (duration approximately 100 seconds) via an indwelling catheter.
[0190]
[0191] *This group of animals underwent all surgical procedures but no cecal manipulation.
[0192] Methods and Experimental Design
[0193] Male Sprague-Dawley obtained from Charles River Canada Inc. St. Constant, Quebec, Canada (Crl: (SD)) Rat (Rattus norvegicus). Animals were allowed to acclimatize to physical and environmental conditions for eight days between harvest and surgical implantation of the catheter. Medication of the animals was started approximately one week after the catheter was surgically implanted to allow the animals to recover properly before handling. Animals were approximately 10-12 weeks old and weighed 327-397 g when treatment be...
Embodiment 3
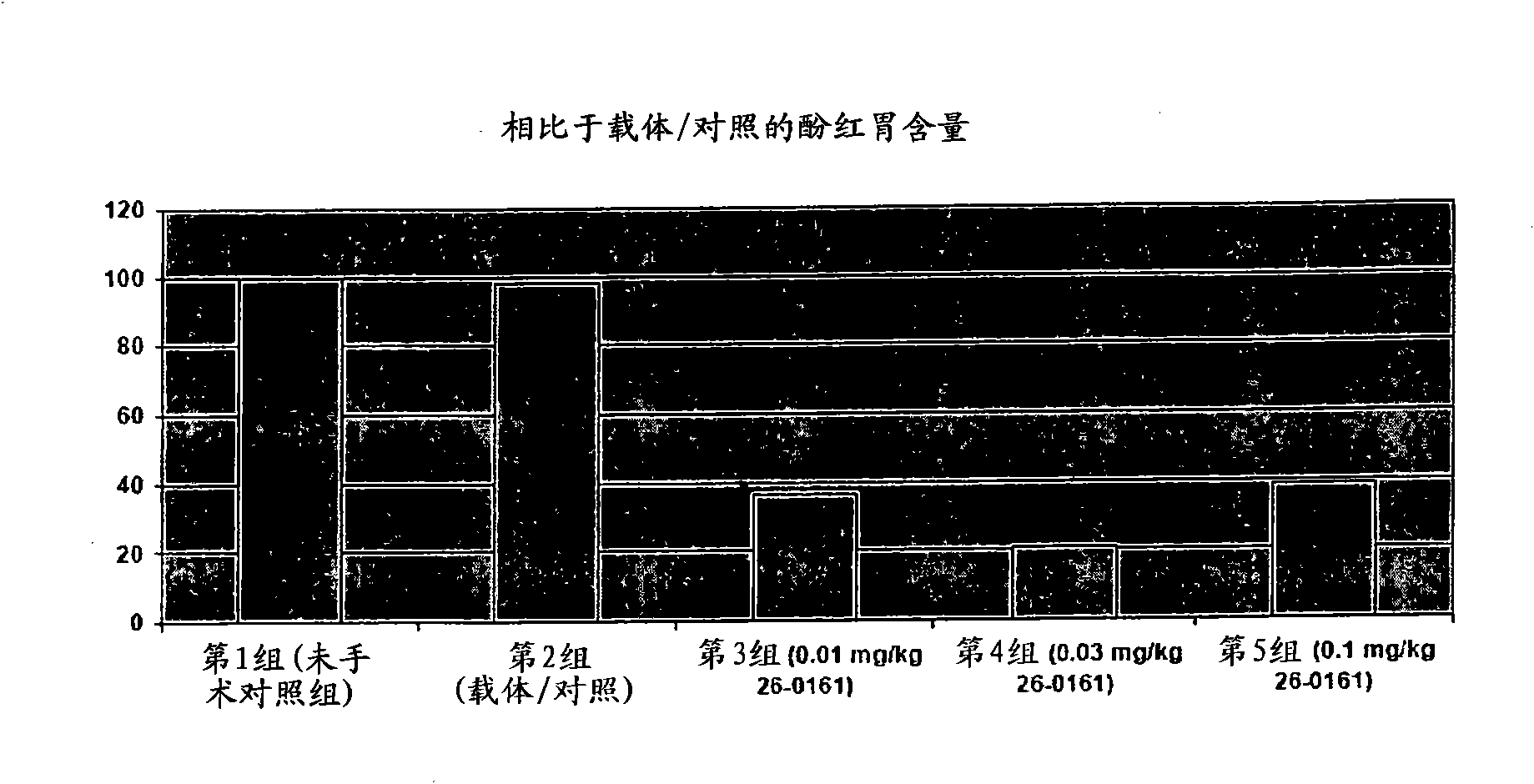
[0213] Example 3. Gastric Kinetic Efficacy of Intravenous Administration of Ipamorelin (0.01, 0.03 or 0.1 mg / kg) in a Rat Model of Postoperative Ileus
[0214] In this study, ipamorelin was administered as a slow bolus intravenous injection (duration approximately 100 seconds) via an indwelling catheter at a dose of 0.01, 0.03, or 0.1 mg / kg.
[0215]
[0216]
[0217] *Animals in this group did not undergo surgery that caused intestinal obstruction.
[0218] Using male Sprague-Dawley (Crl: (SD)) Rat (Rattus norvegicus). The animals were allowed to acclimatize to physical and environmental conditions for seven days between harvest and surgical implantation of the catheter. Medication of the animals was started approximately one week after the catheter was surgically implanted to allow the animals to recover properly before handling. Animals were approximately 10 weeks old with a body weight of 334-385 g at the start of treatment.
[0219] Animals were housed indivi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com