Purified and isolated protein zero related (PZR) and therapeutic and screening methods using same
a technology of purified and isolated proteins and screening methods, applied in the field of purified proteins, can solve problems such as serious deficiency in the art of lack of knowledg
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
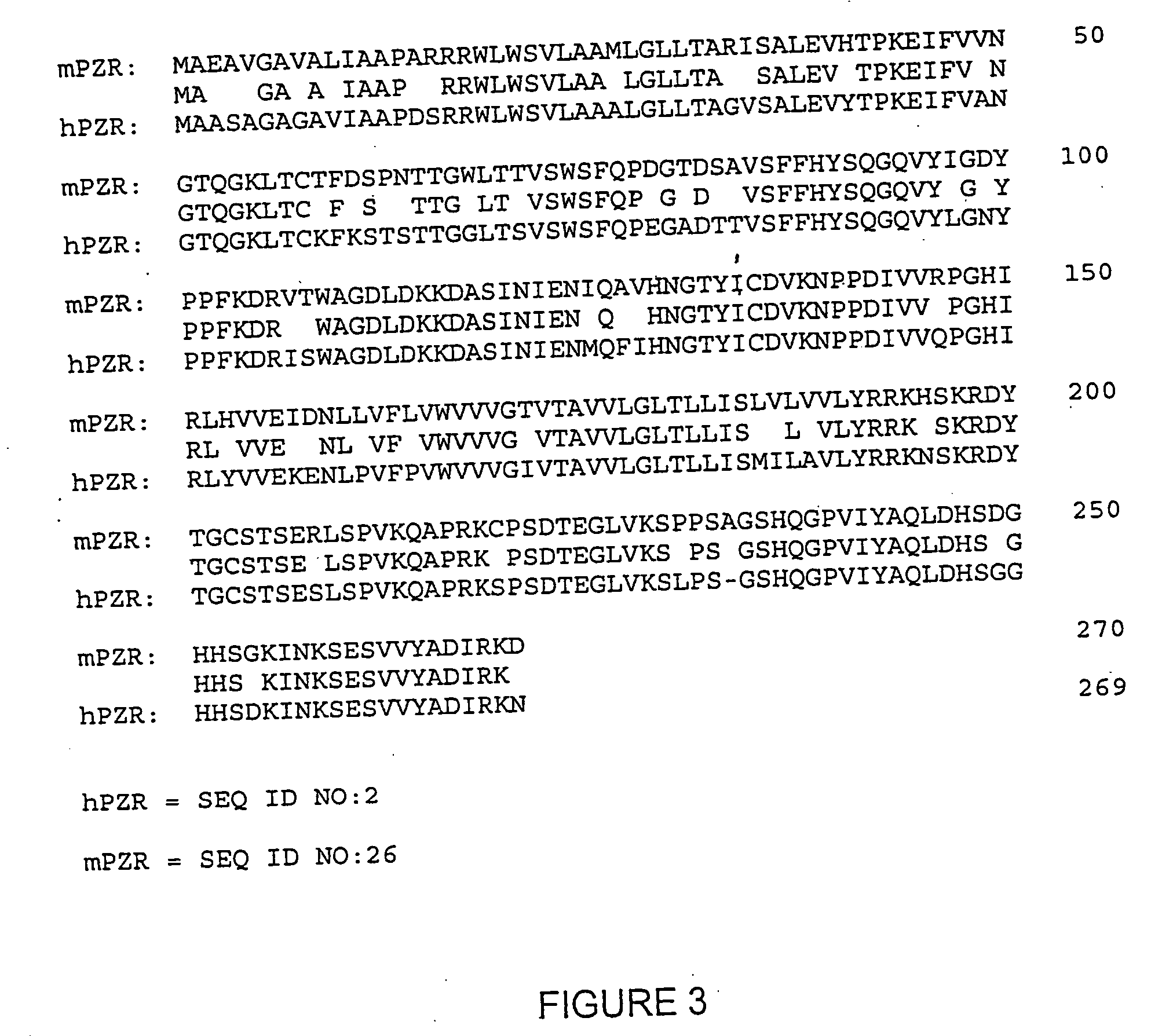
Isolation and Purification of PZR
[0256] Purification of PZR from 293 cells overexpressing the catalytically inactive mutant of SHP-2. The stably transfected 293 cells overexpressing the catalytic inactive mutant of SHP-2 were grown in DMEM / High containing 10% calf serum and 100 unit / ml each of penicillin and streptomycin and 0.25 mg / ml G418 sulfate. After growing to confluency, the cells were treated with 0.1 mM pervanadate for 20 min before harvesting in ice-cold phosphate-buffered saline. The collected cells were broken up with a Dounce glass homogenizer in Buffer A containing 25 mM β-glycerophosphate (pH 7.3), 10 mM EDTA, 2 mM EDTA, 0.2 mM Na3VO4, 1 mM benzamidine, 0.1 mM phenylmethylsulfonyl fluoride, 2 μg / ml leupeptin, 1 μM pepstatin A, and 1 μg / ml aprotinin. Nuclear pellets were removed by centrifugation at 800×g for 20 min, and the remaining postnuclear extract was further centrifuged at 100,000×g for 45 min to give a clear cytosolic supernatant and a pelleted membrane fract...
example 2
Evaluation of the Association of PZR with SHP-2 In Vivo
[0266] Identification and purification of a 43 kDa hyperphosphorylated protein. All PTPs contain a highly conserved cysteinyl residue within their catalytic centers, which is directly involved in the formation of a thiophosphate intermediate essential for the catalysis (reviewed in Fischer, E. H., et al. (1991) Science 253:401-6 and in Walton, K. M. and Dixon, J. E. (1993) Annu. Rev. Biochem. 62:101-20). Mutation of this cysteinyl residue to serine impairs the phosphatase activity. The Cys-to-Ser mutants of SHP-1 and SHP-2 display dominant negative effects and cause hyperphosphorylation of specific cellular proteins on tyrosine as previously described (Zhao, Z., et al. (1995) J. Biol. Chem. 270:11765-17769; Su, L., et al. (1996) J. Biol. Chem. 271:10385-10390. ). In human embryonic kidney 293 cells, expression of the catalytically inactive Cys-to-Ser mutant form of SHP-2 resulted in tyrosine phosphorylation of 43 and 95 kDa pro...
example 3
Expression of a Soluble PZR Polypeptide
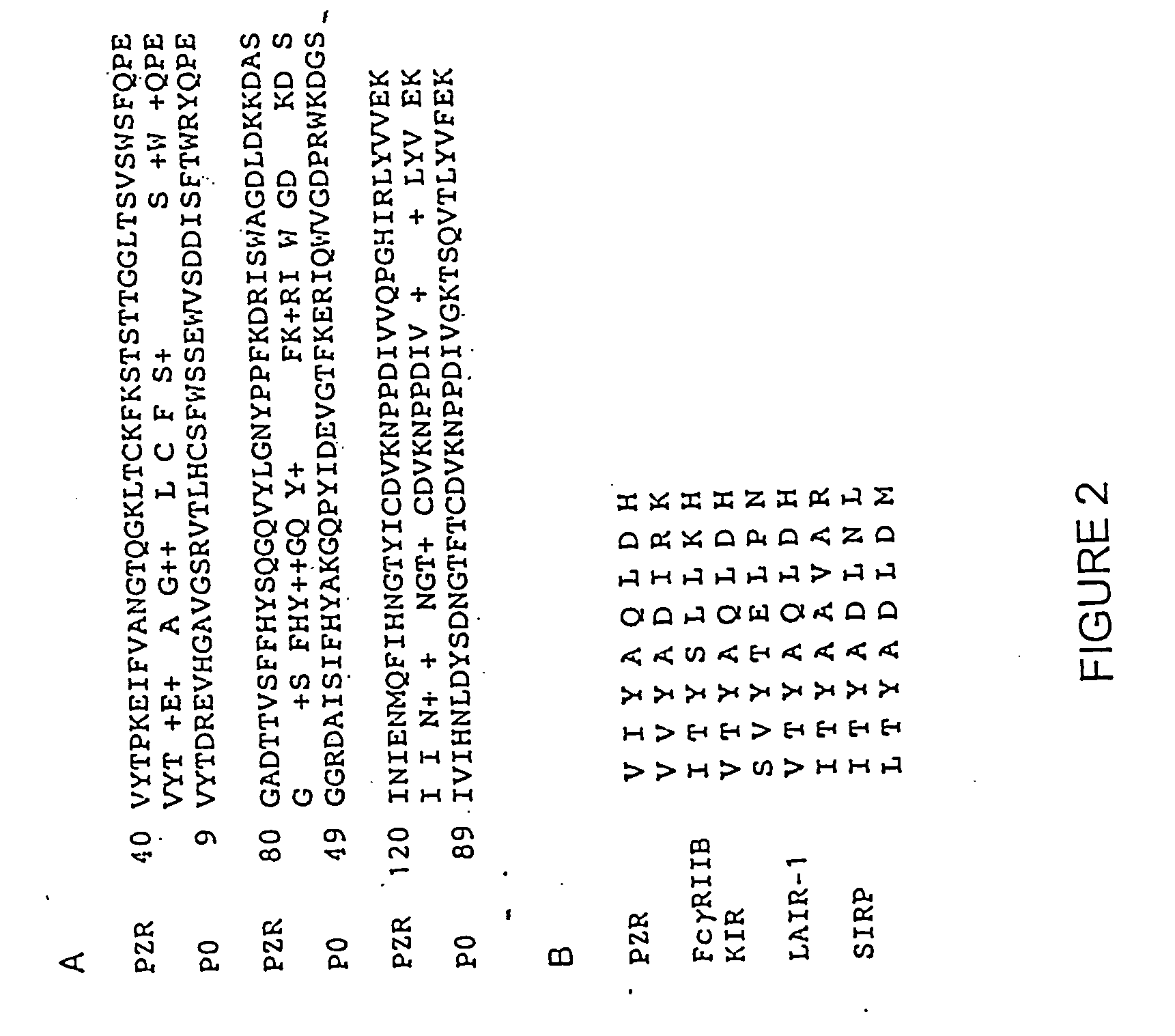
[0286] The expression of a soluble form of PZR was performed in 293 cells. The cDNA encoding the signal sequence and the extracellular portion of PZR was cloned into the pCDNA3 vector, and the DNA construct was used to transfect 293 cells. The culture medium was analyzed for expression of soluble PZR by using Western blotting with an anti-PZR antibody. Three forms of soluble PZR representing different degrees of glycosylation were seen at 14.4, 21.5 and 29 kDa, respectively. Representative amino acid and nucleic acid sequences for soluble PZR are set forth in SEQ ID NOs:33-40.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com