Use of cell lines to produce active therapeutic proteins
a cell line and active technology, applied in the field of cell lines, can solve the problems of perceived risks, not all behave identically to their native counterparts, and manufacturers, users, and patients, and achieve the effect of enhanced expression and enhanced expression of the naturally occurring gene encoding the protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Characterization of Immortalized Human Hepatocytes
[0233] Over 100 human hepatocyte clonal cell lines were established by transfecting human hepatocytes with the simian virus 40 large T and small t antigen genes under control of the SV40 early promoter. Two cell lines designated Ea1C-35 and Fa2N-4 are described.
[0234] Both cell lines were created by lipofection-mediated transfection of primary cryopreserved human hepatocytes with vectors containing the SV40 largeT and small t antigens. The Ea1C-35 cell line was derived from transfection of cryopreserved human hepatocytes with the immortalization vector pBlue Tag, a recombinant plasmid containing the early region of wild-type SV40. The pBlue Tag vector was constructed as follows: pBR / SV (ATCC) was digested with restriction enzymes KpnI and BamHI to release a 2995 bp fragment (239-2468 bp, numbering according to Fiers, W et al Science, 273:113-120) containing the SV40 early promoter and the coding regions from small t and large T ant...
example 2
Expression of Liver Specific Transcription Factors
[0236] Since retention of liver specific transcription factors is a prerequisite for expression of hepatic functions, clonal cell lines were initially screened by RT-PCR using primers for human HNF1, HNF3, HNF4α, HNF4,γ and C / EBP and albumin. Briefly, total RNA was prepared from 106 cells of each clonal cell line using the micro-isolation method of Brenner et al. (55). Where is the information for the previous reference? 50 μg of E. coli rRNA (Sigma) was used as a carrier to facilitate the isolation of RNA from a small number of cells. RT-PCR reactions were carried out using the Perkin Elmer Cetus, GeneAmp RNA PCR Kit. One μg of total RNA was reverse transcribed using random hexamers and M-MLV reverse transcriptase according to the supplier's instructions. The PCR reaction was carried out using oligonucleotide primers that defined nucleotide fragments unique for each transcription factor. The primers were commercially synthesized an...
example 3
SV40 Mediated Proliferative Activity
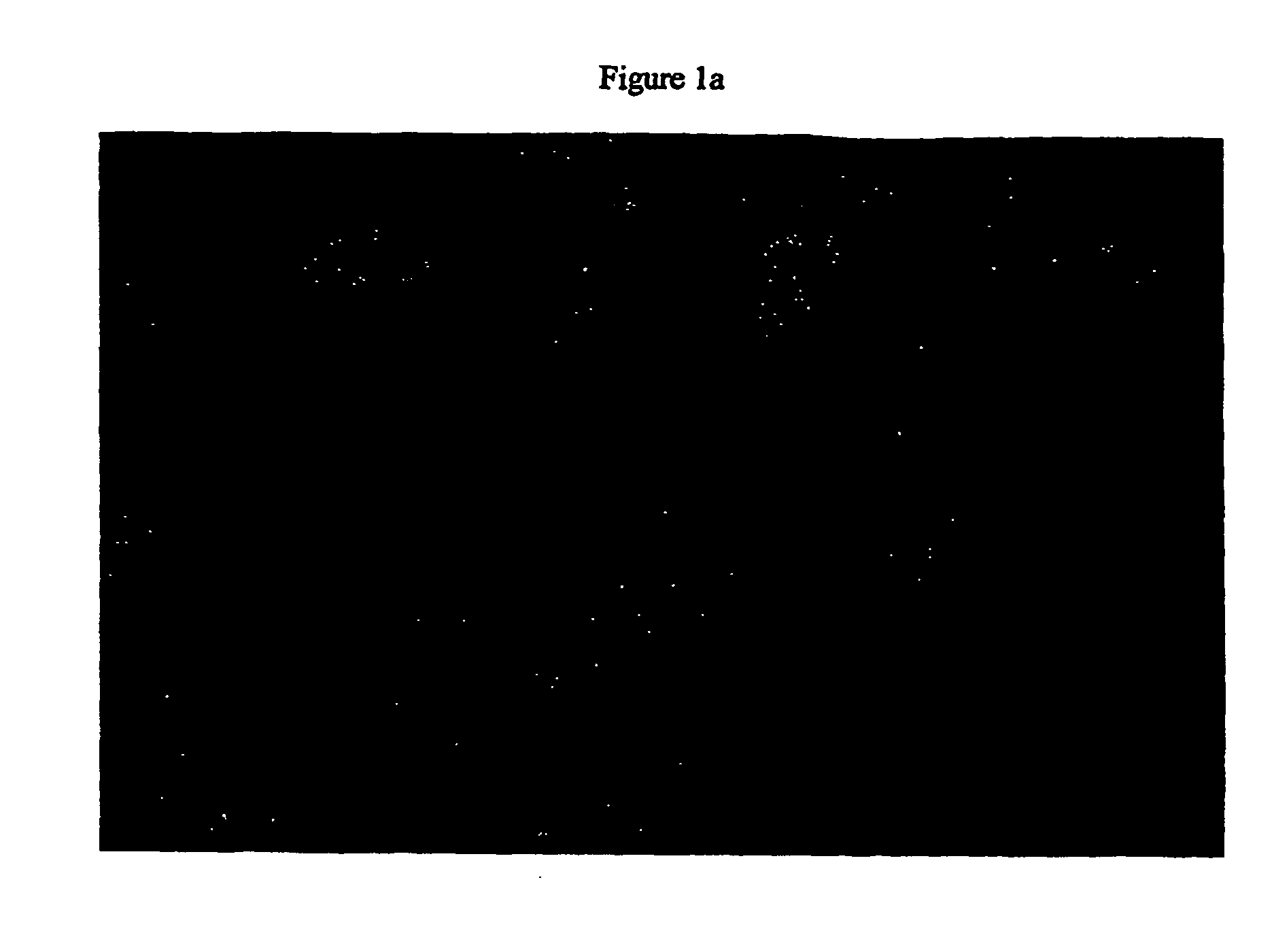
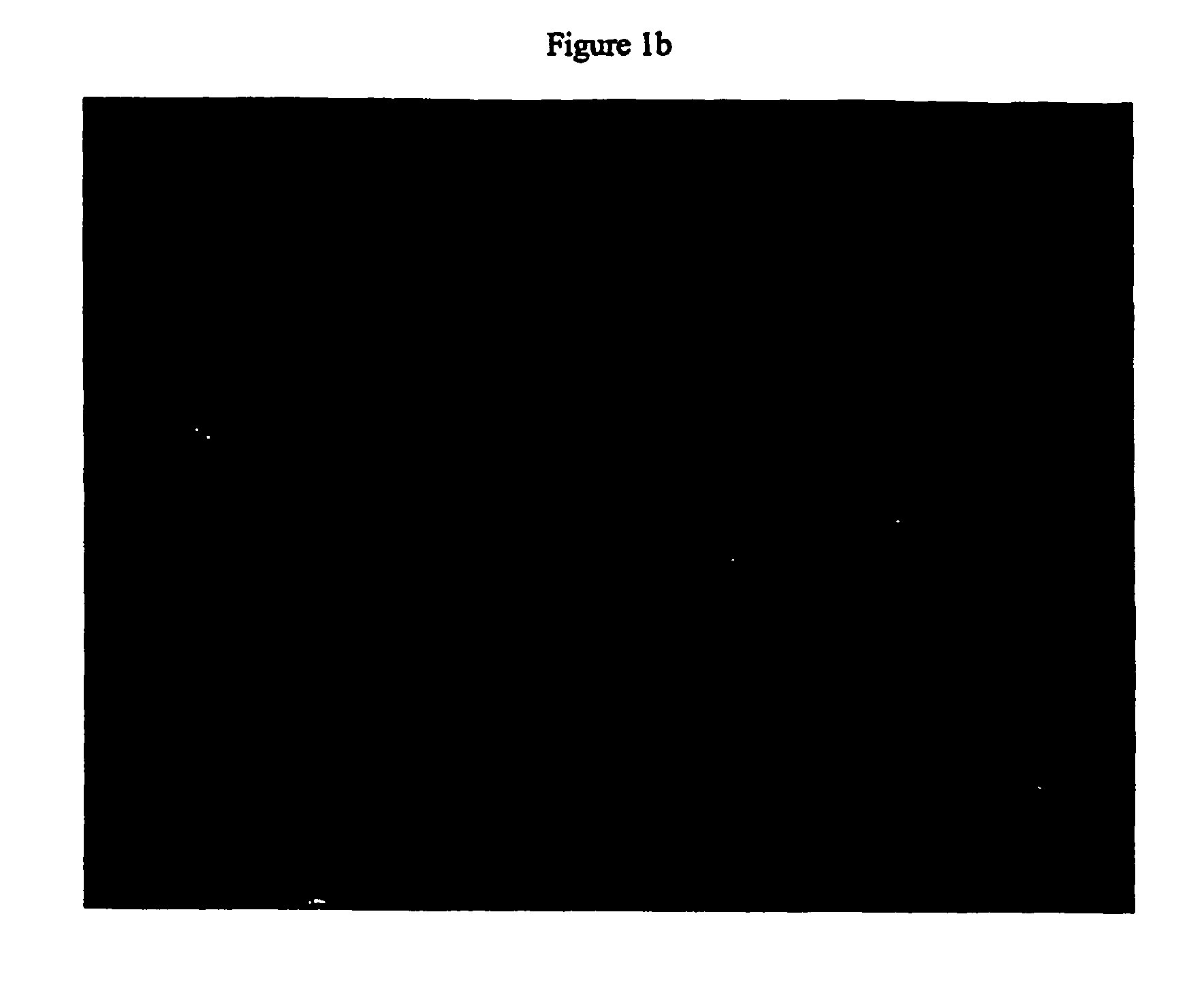
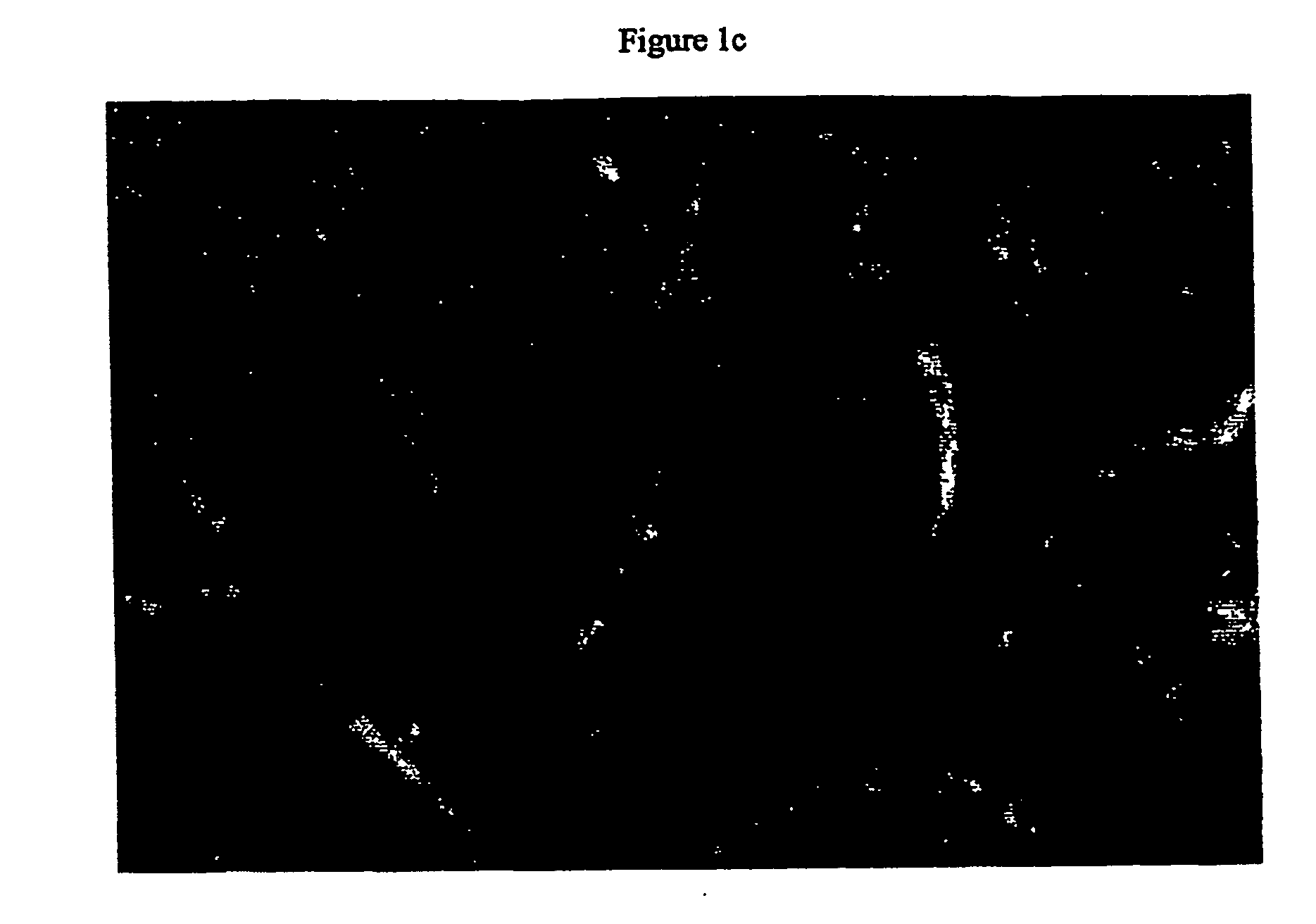
[0237] Primary human hepatocytes have limited proliferative activity when cultured. In order to overcome this characteristic, SV40 large T and small t antigens were introduced into the genome. The resulting clonal cell lines, Fa2N-4 and Ea1C-35 have subsequently been maintained in culture for up to 18 months. Both immortalized lines grow and function when maintained in MFE medium and can be cryopreserved and banked. Indirect immunofluorescent staining using polyvalent antibodies against large T antigen and albumin demonstrated that the cell lines continue to express the nuclear localized immortalizing gene FIG. 1a) as well as express a hepatocyte specific gene characteristic of differentiated function (FIG. 1b). The morphology of the Ea1C-35 cell line is shown below (FIG. 1c).
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com