Methods, compositions, and kits for the detection of bacteria in a sample
a technology for applied in the field of analytical methods of detecting the presence of bacteria in a sample, can solve the problems of requiring long incubation times, bacterial contamination of transfusion products known as a potential source of harm, and the incidence of illness and fatalities caused by bacterial contamination, and the frequency of bacterial contamination of blood platelets, and the effect of increasing the number o
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
ELISA Detection of Bacteria using an Anti-Peptidoglycan Antibody
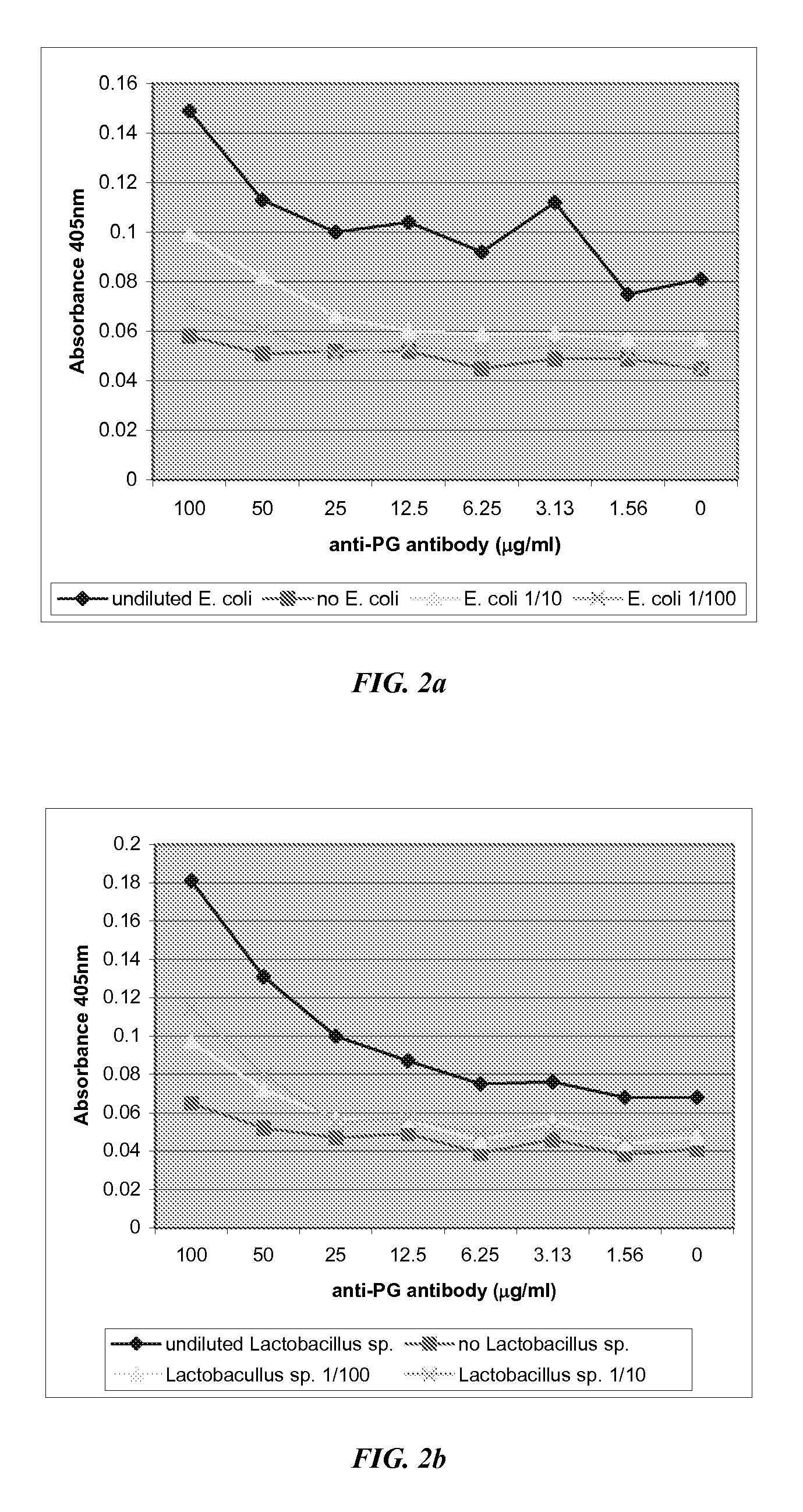
[0089]This example demonstrates that monoclonal antibodies against peptidoglycan can be used to detect the presence of gram positive and gram negative bacteria in liquid samples. FIG. 1 shows the results of an ELISA using an anti-peptidoglycan monoclonal antibody obtained from Chemicon International, (Temecula, Calif.) to detect Lactobacillus sp. and E. coli strain 392. It was also demonstrated that antibodies directed against NAG and NAM could be successfully used according to the invention.
[0090]These organisms were examined to determine if peptidoglycan antibodies detect their presence in a standard ELISA format. The procedure was carried out using a method as follows.[0091]1. A 1 ml sample of bacteria was washed 3× in PBS and resuspended in 1 ml of PBS for a final concentration of approximately 1×108 cfu / ml. This was then diluted ten-fold eight times.[0092]2. Each well received 100 ul of the appropriate dilution of ...
example 2
Detection of Bacteria using an Anti-Peptidoglycan Antibody in a Lateral Flow Immunological Assay
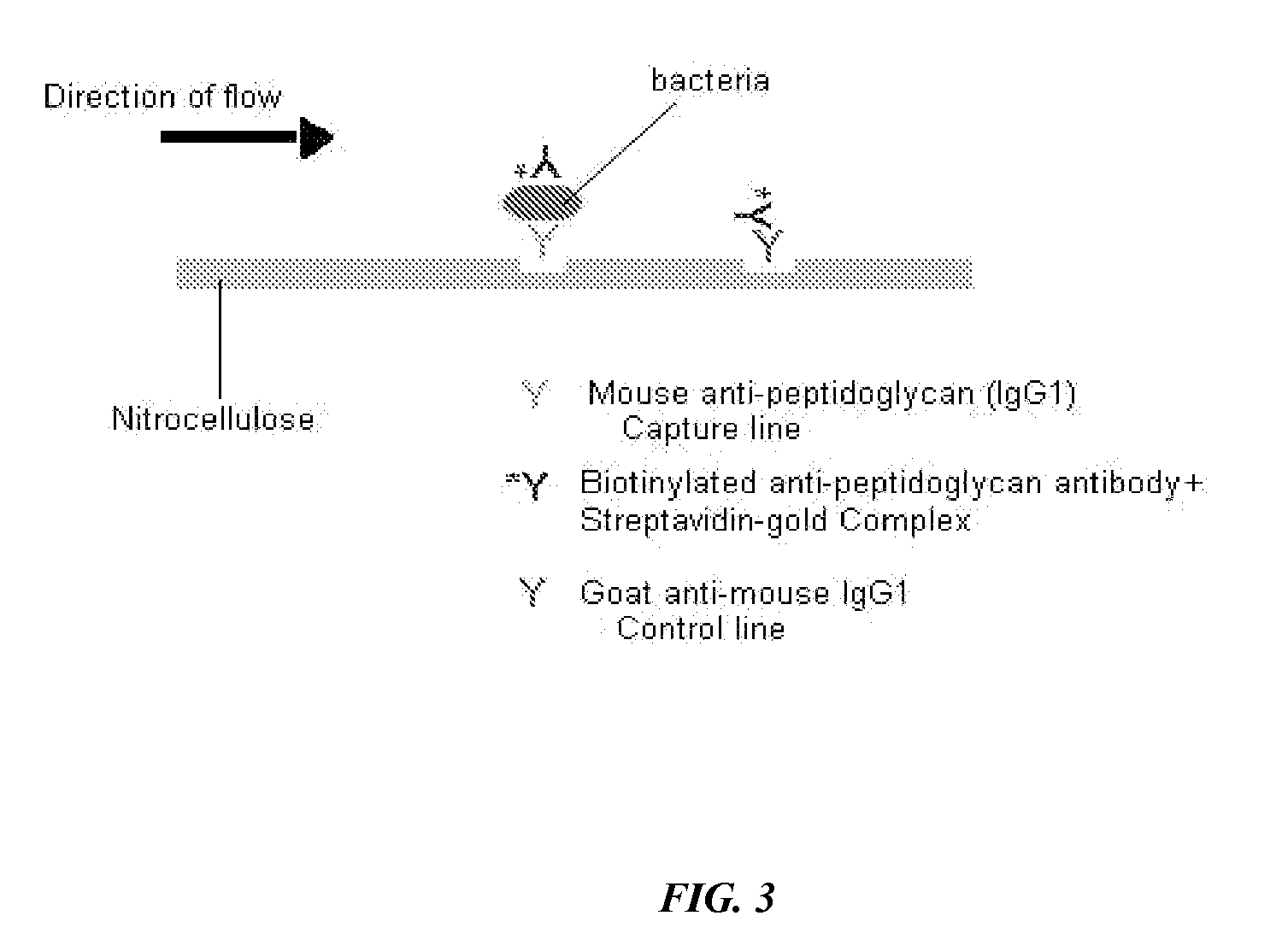
[0105]This example demonstrates that bacteria can be detected in a liquid sample using a lateral flow immunological sample. Schematic diagrams of the principle of the lateral flow assay devised during this project and an exemplary lateral flow detection device are shown in FIGS. 3 and 4, respectively.
[0106]Lateral flow immunological assays were performed as depicted to optimize the relative concentrations of antibody-labeled conjugates for lateral flow detection. The purpose of these experiments was to determine the optimum concentrations of the various conjugates for detecting bacteria in the threshold range of 5×103 organisms / ml. Various concentrations of the capture antibody, biotin-labeled antibody and streptavidin-gold conjugate were tested in order to optimize the assay. Labeled antibodies were prepared using BiotinTag Micro-biotinylation Kit, Catalog B-Tag from Sigma Chemical Co., ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com