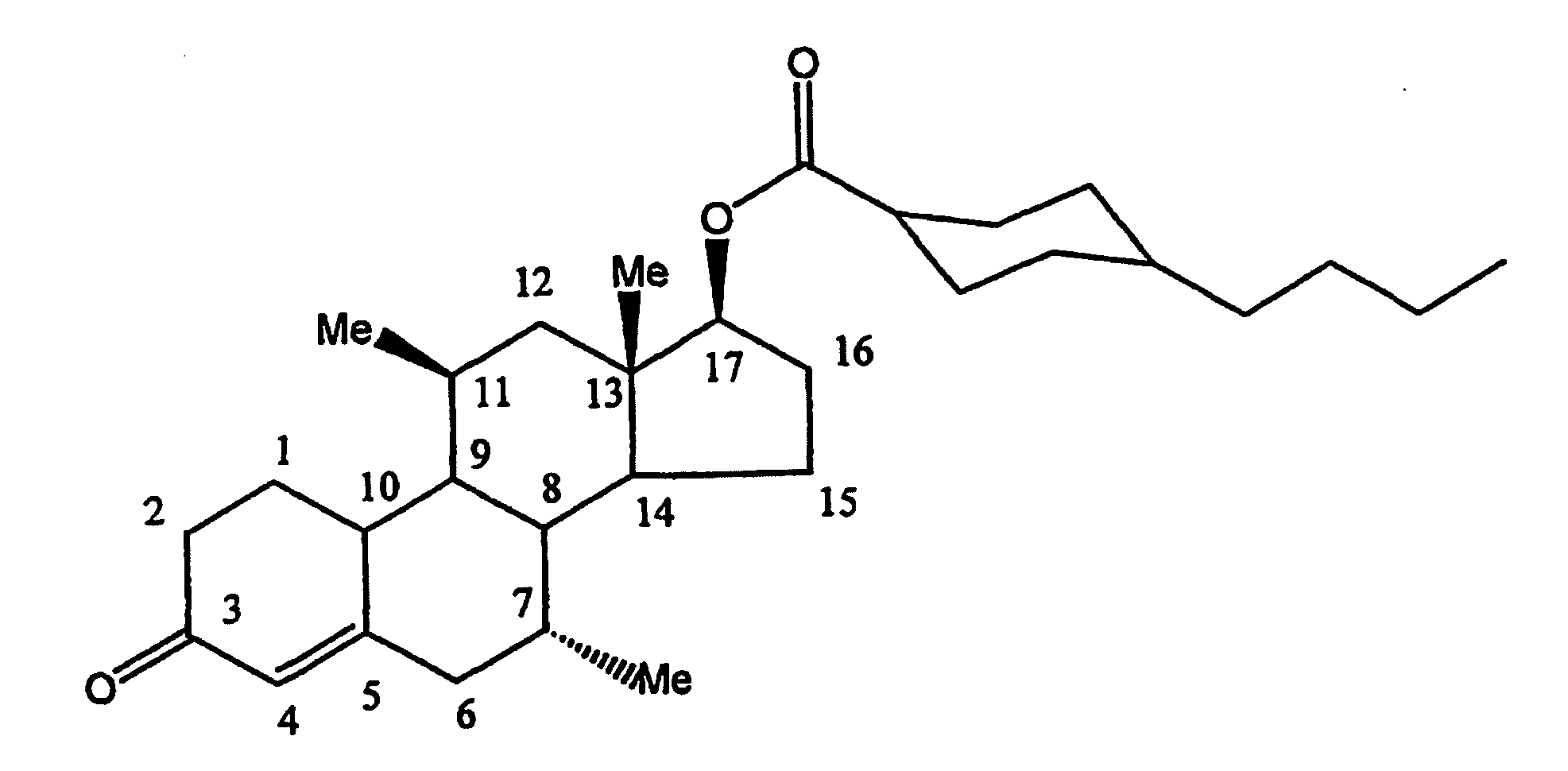
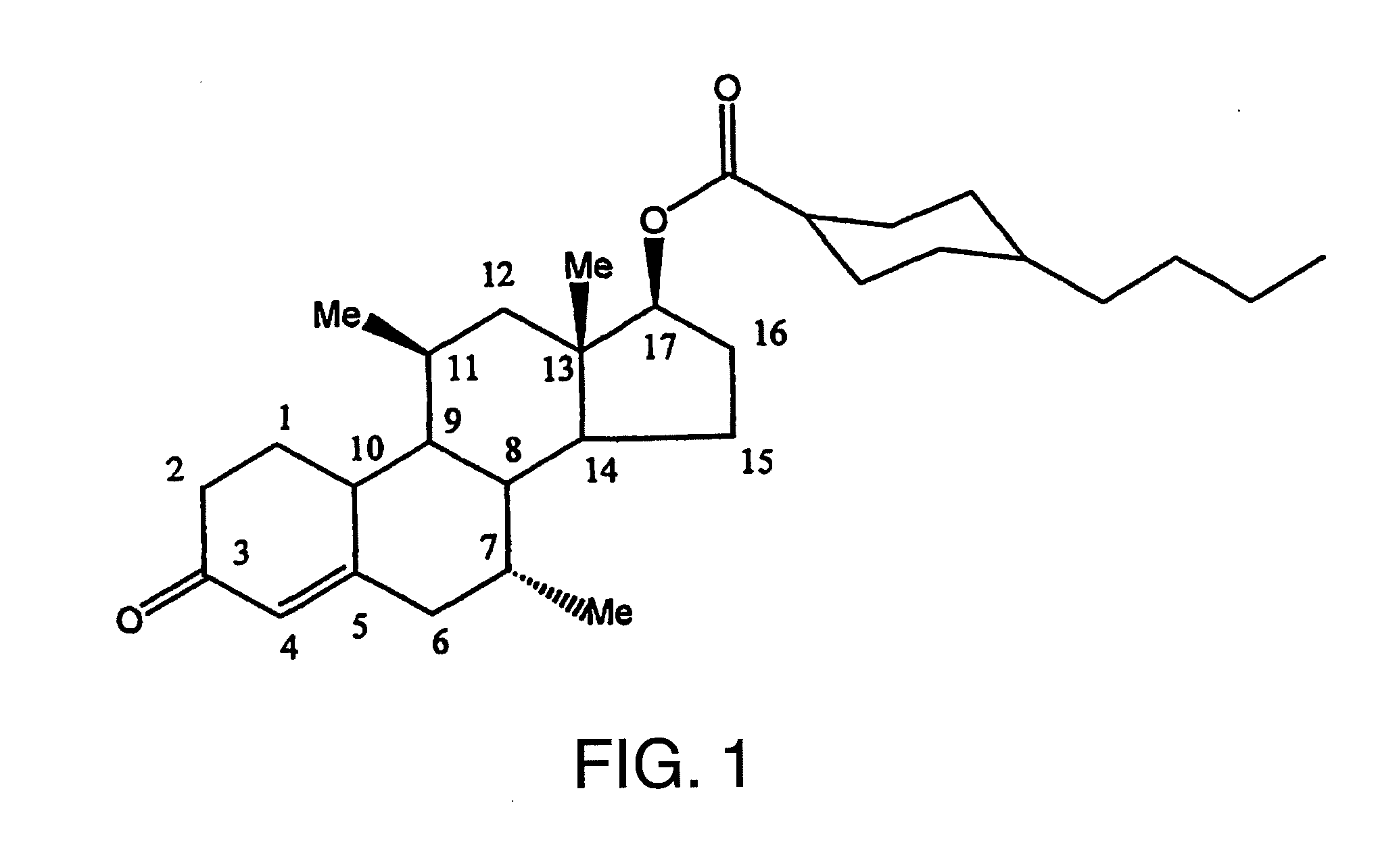
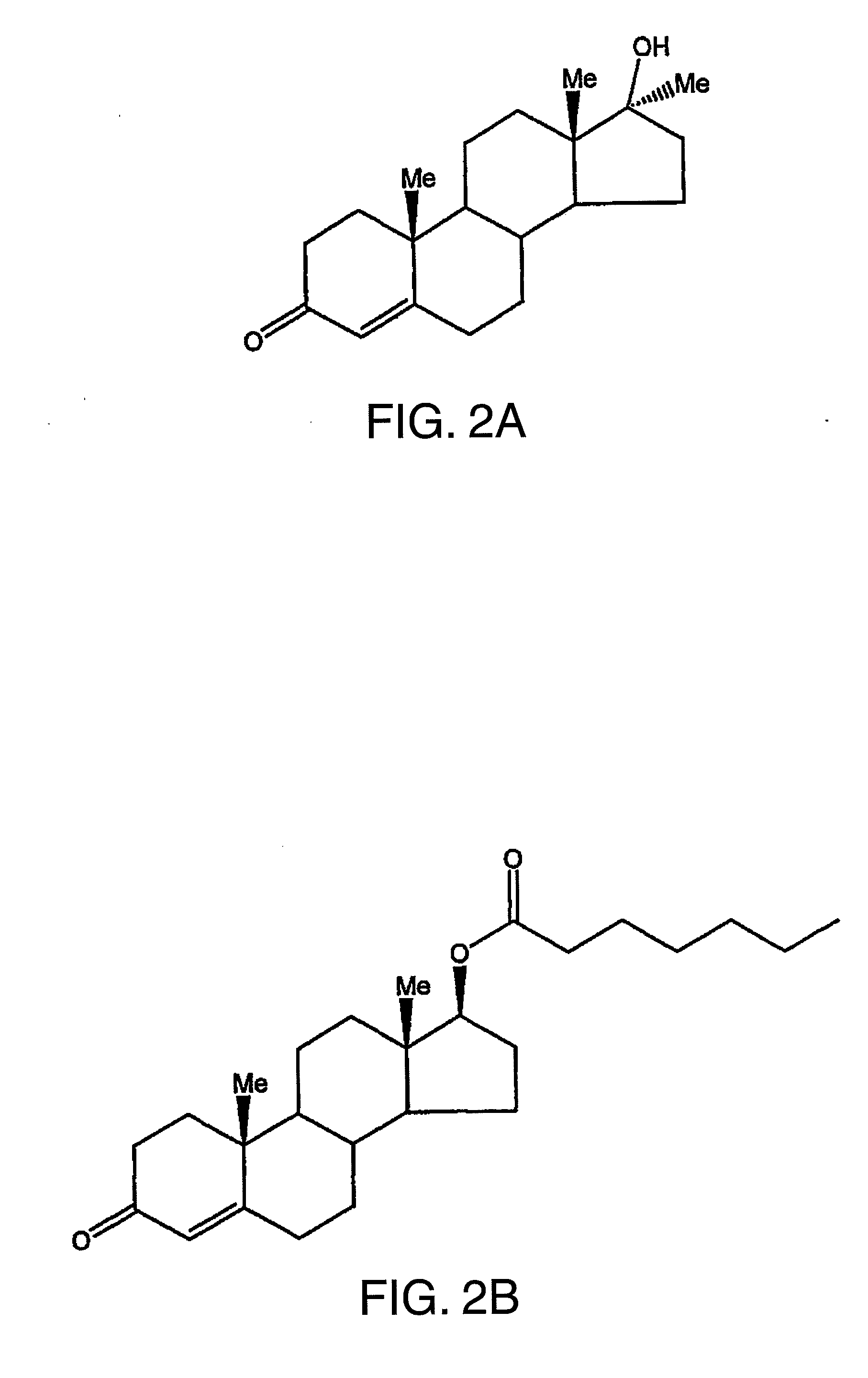
METHOD OF MAKING AND USING 7alpha,11beta-DIMETHYL-17beta-HYDROXYESTR-4-EN-3-ONE 17-UNDECANOATE
a technology of androgen esters and esters, which is applied in the field of making and using androgen esters, can solve the problems of difficult therapy, and unpredictable activity of androgen esters, and achieves positive affecting the duration of activity and yield. high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0075]This example provides data on the androgenic potency of 7α,11β-dimethyl-17β-hydroxy-4-estren-3-one bucyclate (CDB-4386A), its free alcohol (CDB-1321D), testosterone bucyclate (CDB-1781V-1), methyltestosterone (CDB-110), testosterone (CDB-111C) and testosterone enanthate (CDB-112a) when administered orally.
[0076]Immature (about 21-day-old) Sprague-Dawley rats were orchidectomized under anesthesia, and randomly assignee to groups often animals for each dose level of the active undergoing testing. Each active was dissolved in 10% ethanol / sesame oil and administered by gavage (oral) each day for seven days beginning on the date of the orchidectomy. The animals were sacrificed 24 hours after the last dose, and the ventral prostate and seminal vesicles were excised, cleaned of fat and connective tissue, blotted on moist filter paper and weighed to the nearest 0.1 mg. See, e.g., Hershberger, L. et al, Myotrophic Activity of 19-nortestosterone And Other Steroids Determined By Modified...
example 2
[0078]This example provides data that demonstrates the duration of activity of 7α,11β-dimethyl-17β-hydroxy-4-estren-3-one bucyclate (CDB-4386A) compared to its free alcohol (CDB-1321D), the 11α-methyl analog of 7α,11β-dimethyl-17β-hydroxy-4-estren-3-one bucyclate (CDB-4386), testosterone bucyclate (CDB-1781a, -1781V2), and testosterone enanthate (CDB-112E) when administered parenterally (by subcutaneous injection).
[0079]Immature (about 21-day-old) Sprague-Dawley rats were orchidectomized under anesthesia, and randomly assignee to groups of ten animals for each dose level of the active undergoing testing. Each active was administered by subcutaneous injection each day for seven days beginning on the date of the orchidectomy. The animals were sacrificed 24 hours after the last dose, and the ventral prostate and seminal vesicles were excised, cleaned of fat and connective tissue, blotted on moist filter paper and weighed to the nearest 0.1 mg. Regression analysis was performed by conve...
example 3
[0085]This example illustrates the relative androgenic activity of testosterone and its derivatives.
[0086]Immature (about 21-day-old) Sprague-Dawley rats were orchidectomized under anesthesia, and randomly assignee to groups of ten animals for each dose level of the active undergoing testing. Each active was dissolved in 10% ethanol / sesame oil and administered by gavage (oral) or subcutaneous injection each day for seven days beginning on the date of the orchidectomy. The animals were sacrificed 24 hours after the last dose, and the ventral prostate and seminal vesicles were excised, cleaned of fat and connective tissue, blotted on moist filter paper and weighed to the nearest 0.1 mg. Regression analysis was performed by conventional methods using a PROPHET data management system. Ventral prostate weight was used as the endpoint because it is the sensitive organ to androgenic stimulation.
[0087]FIGS. 6 and 7 are graphic representations of the androgenic assays of the actives. Each da...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap