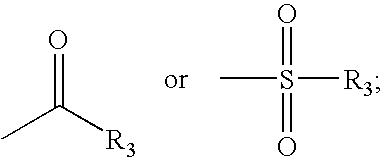Dual modulators of 5HT2a and d3 receptors
a technology of d3 receptor and modulator, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, biocide, etc., can solve the problems of reduced patient quality of life and socioeconomic problems, less effective treatment of negative symptoms, and reduced patient compliance, and achieves high affinity for d3
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
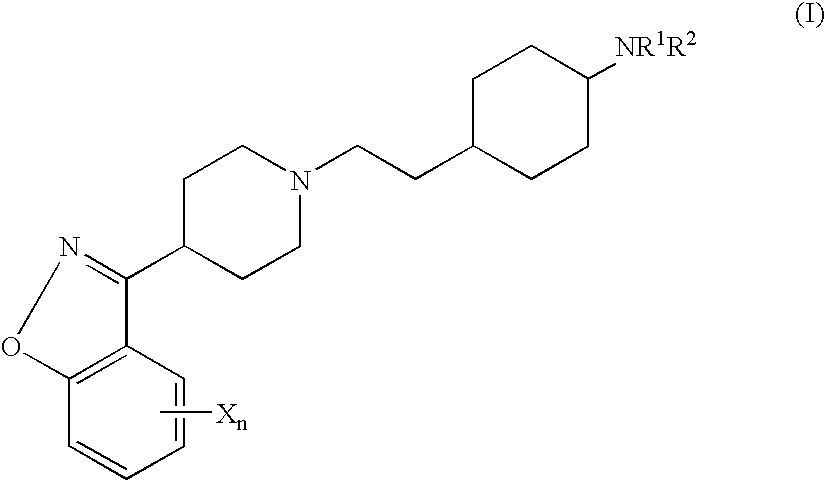
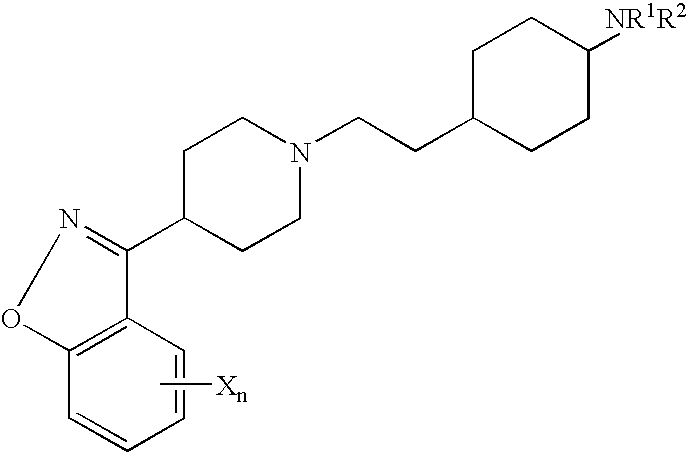
4N-trans(4-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-cyclohexyl)-acetamide
Intermediate B
Trans-(4-amino-cyclohexyl)-acetic acid ethyl ester
Step 1
[0358](4-Nitro-phenyl)-acetic acid (50g, 276 mmol) was added to a stirred solution of 22.08g of 50% sodium hydroxide solution in 450 mL deionizated water. The clear yellow solution is transferred into a high-pressure autoclave that it charged with 30 g (511 mmol) of water-wet sponge nickel catalyst. The autoclave is sealed, flushed with nitrogen and then pressurized to 115 bar with hydrogen. The reaction mixture is stirred and heated to 125° C. for 48 h. At that time the autoclave is cooled, vented and charged under nitrogen with another 30 g (511 mmol) of the sponge nickel catalyst. The autoclave is flushed again with nitrogen and then pressurized to 115 bar and the vessel is heated to 130° C. while stirring (a maximum pressure of 130 bars is observed). Hydrogenation is continued for 5 days to 130° C. The autoclave is t...
example 2
Tetrahydro-pyran-4-carboxylic acid trans(4-{2-[4-(6-fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-cyclohexyl)-amide
[0365]Tetrahydro-pyran-4-carboxylic acid (0.013 g, 0.096 mmol), 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyl uronium tetrafluoroborate (0.026 g, 0.08 mmol) and (0.04 mL, 0.24 mmol) of N-ethyldiisopropylamine were stirred in 0.6 mL of DMF for 0.5 h at room temperature and Trans 4-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-cyclohexlamine (could be obtained as the trifluoroacetic acid salt) (trifluoro acetic acid salt) (0.030 g, 0.08 mmol) was added. The mixture was stirred for 12 hours at room temperature. The mixture was concentrated to dryness and the residue was taken up on methanol and purified with preparative HPLC on reversed phase eluting with acetonitrile / water. The combined producted fractions were evaporated under reduced pressure to yield 0.027 g of a off-white solid (0.06 mmol, 74%). MS (m / e): 458.5 (M+H+).
[0366]According to the proce...
example 24
N-trans(4-{2-[4-(5-Fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-cyclohexyl)-acetamide
Intermediate E
Trans (4-{2-[4-(5-Fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-ethyl}-cyclohexyl)-carbamic acid tert-butyl ester
[0367]A mixture of 5-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole (1.3 g, 5 mmol), trans-[4-(2-Oxo-ethyl)-cyclohexyl]-carbamic acid tert-butyl ester (example 1, intermediate C) (1.6 g, 6 mmol), triethylamine (0.64 mL, 5 mmol) in 1, 2 dichloroethane (27 mL) was stirred for 4 h at room temperature and sodium triacetoxyborohydride (1.9 g, 9 mmol) was added slowly and the resulting solution was stirred for 12 hours until the TLC indicated completion of the reaction. The mixture was filtrated and concentrated to dryness and purified with column chromatography on silica gel using CH2Cl2—CH2Cl2 / MeOH (1-9:1). The product fractions were concentrated to give 2.3 g (5.1 mmol, 100% yield) of a off-white solid. MS (m / e): 446.3 (M+H+).
Intermediate F
Trans 4-{2-[4-(5-Fluoro-benzo[d]is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap