Antagonists of the bradykinin B1 receptor
a technology of bradykinin and b1 receptor, which is applied in the field of anti-bradykinin b1 receptor antagonists, can solve the problems of life style alteration, debilitating and/or dangerous side effects, ulceration, bleeding, etc., and achieves the effect of superior pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis and Purification of B1 Receptor Peptide Antagonists and PEG-Conjugated B1 Receptor Peptide Antagonists
[0224]Various peptides of the invention were synthesized using synthesis techniques well-known in the art. A preferred method of synthesizing various peptides of the invention uses a FMOC strategy with carbodiimide activation as described below.
[0225]Part 1: Dissolve Fmoc-Amino Acid to Resin Using Carbodiimide Chemistry.
[0226]Fmoc-amino acid (3-4 equivalent) was dissolved in dry DCM / NMP mixture (NMP or DMF was used to aid complete dissolution). A solution of N-hydroxybenzotriazole (HOBt, same equivalent to amino acid) in NMP was added to the amino acid solution. A solution of N,N′-dicyclohexyl-carbodiimide (DCC, same equivalent to amino acid) in DCM was added to the amino acid solution. The solution was mixed for approximately 20 minutes. The activated acid solution was then added to resin (if needed, precipitates were removed prior to addition). The reaction was agitated ...
example 2
Synthesis and Purification of PEG-Conjugated B1 Receptor Peptide Antagonists Using PEG Thioesters
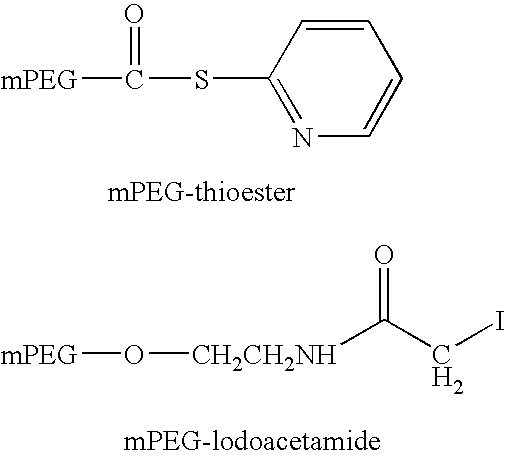
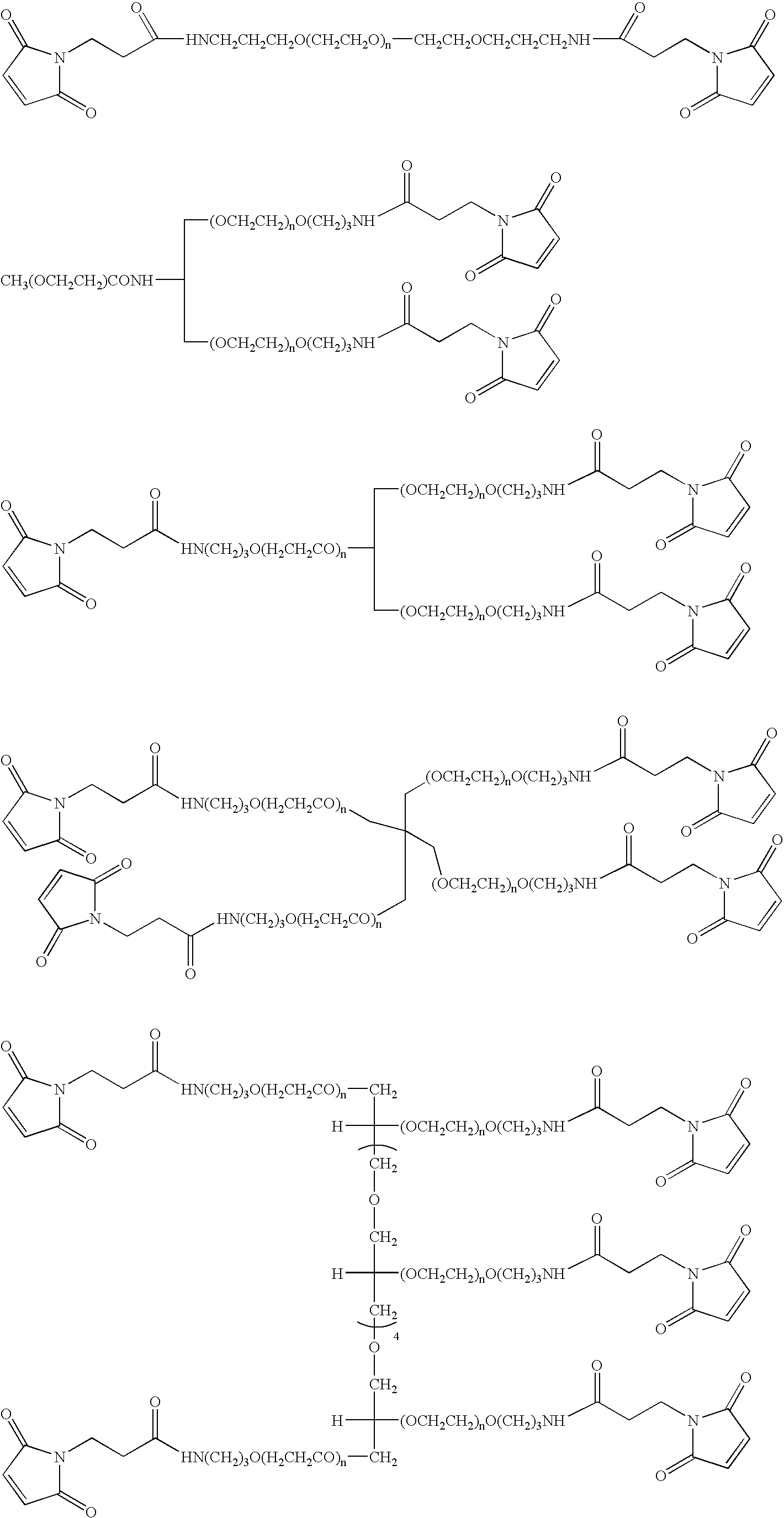
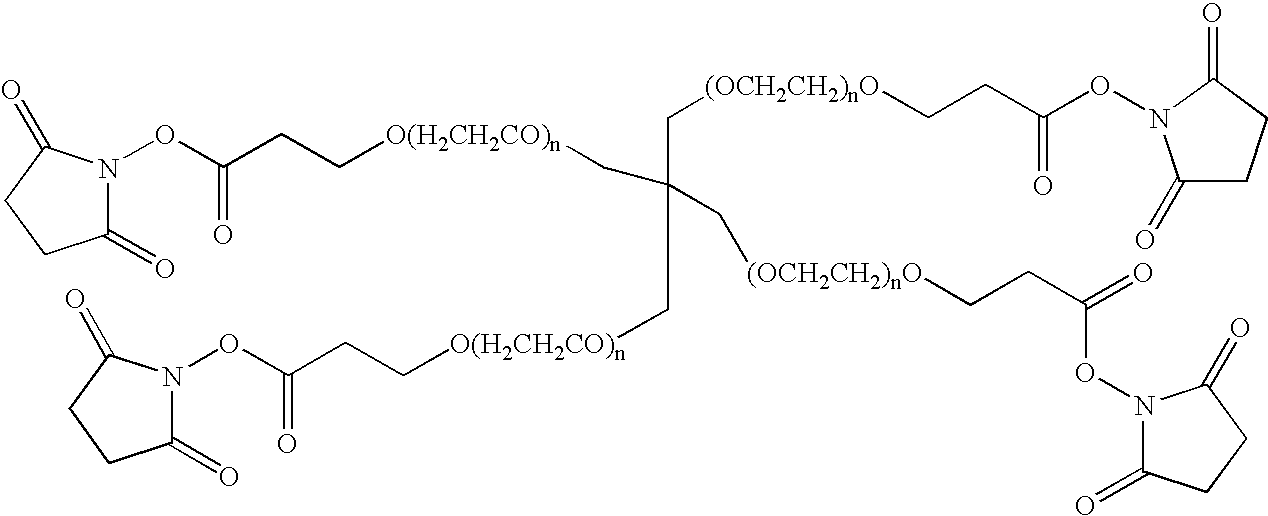
[0246]PEG-conjugated peptides were prepared by reacting a cysteine containing peptide with PEG-maleimide in 50 mM NaHPO4, 5 mM EDTA, pH 7 at 2.5-5 mg / ml peptide and a reaction stoichiometry of 1.2-fold molar excess of maleimide:thiol. The reaction was stirred at room temperature (20-25° C.) for 18-26 hours. Once complete, the reaction is quenched with a 10-fold molar excess of β-mercaptoethanol (β-ME):maleimide and allowed to stir an additional 30-60 minutes at room temperature. The reactions were purified as described for Method A in Example 1 above.
example 3
Synthesis and Purification of PEG-Conjugated B1 Receptor Peptide Antagonists Using PEG Thioesters or Iodoacetates
[0247]PEG-conjugated peptides were prepared by reacting peptide containing a N-terminal cysteine residue with PEG-OPTE (ortho-pyridyl thio ester) in 50 mM NaHPO4, 5 mM EDTA, pH 7 at 2.5-5 mg / ml peptide and a reaction stoichiometry of 1.2-fold molar excess of activated PEG:peptide. The reaction was stirred at room temperature (20-25° C.) for 18-26 hr. Once complete, the reaction is quenched with a 10-fold molar excess of cysteine:excess PEG reagent and allowed to stir an additional 30-60 minutes at room temperature. The reactions were purified as described in Method A of Example 1 above.
[0248]Alternatively, PEG-iodoacetamide may be used as described above to form conjugates in which the PEG moiety is attached via a thioether linkage (Scheme 3). In this case 1.5 molar equivalents of the activated PEG reaction is used and the reaction time is increased to 24 hours the reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| threshold | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com