Compositions and methods for detection of antibody binding to cells
a technology of antibody binding and cell, applied in the field of binding proteins, can solve the problems of human antibody in vitro, ebv)-mediated transformation or cell fusion, and many proteins cannot be purified in a non-denatured sta
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Cell Surface-Specific Human Monoclonal Antibodies Using Phage Display and Magnetically-Activated Cell Sorting
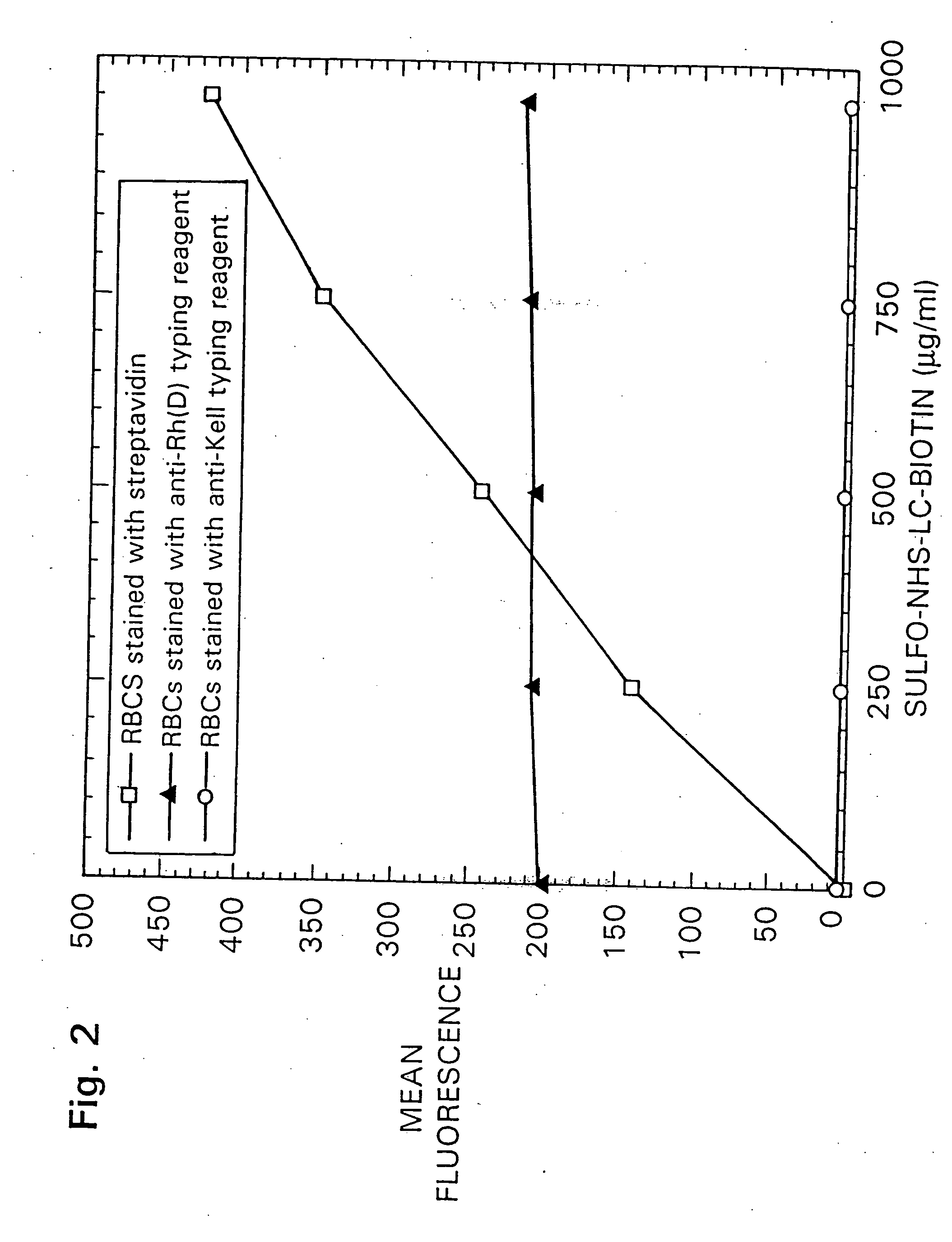
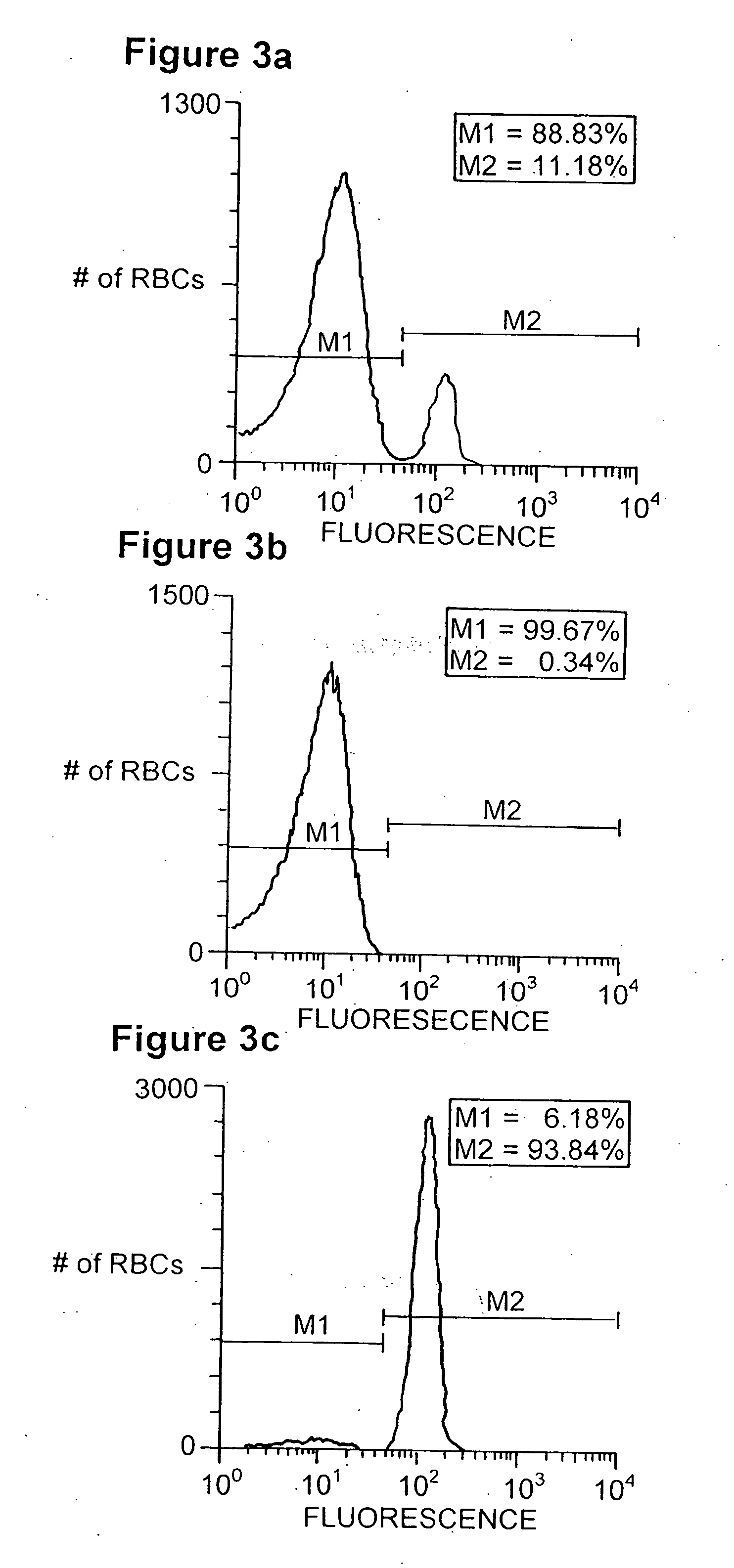
[0120] The experiments described in this Example provide procedures and results for the isolation and production of anti-Rh(D) red blood cell antibodies using Fab / phage display.
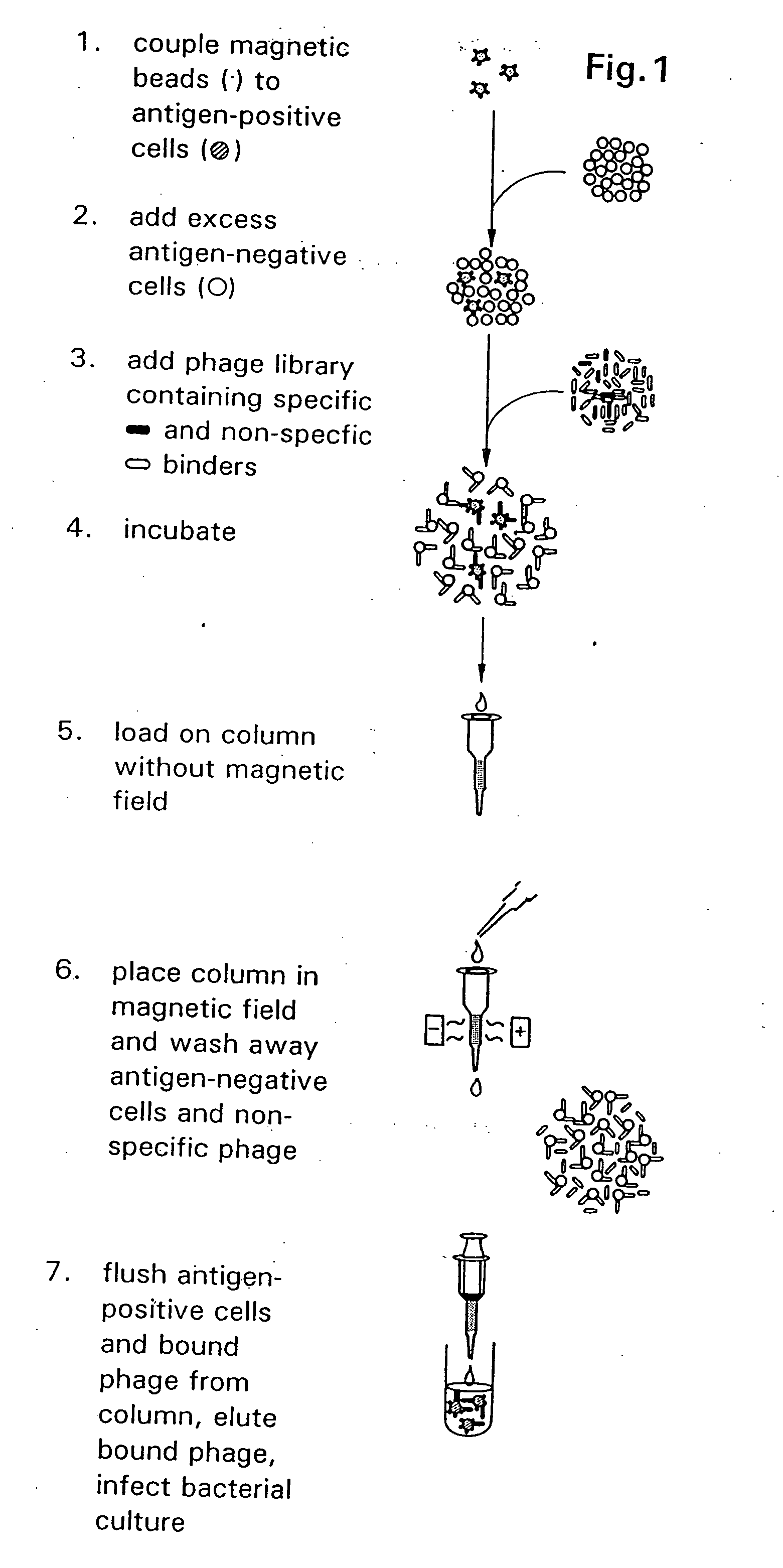
[0121] A method is described in FIG. 1 for the isolation of filamentous phage-displayed human monoclonal antibodies specific for non-purifiable cell surface expressed molecules. To optimize the capture of antigen-specific phage and minimize the binding of irrelevant phage antibodies, a simultaneous positive and negative selection strategy was employed. Cells bearing the antigen of interest are pre-coated with magnetic beads and are diluted into an excess of unmodified antigen-negative cells. Following incubation of the cell admixture with a Fab / phage library, the antigen positive cell population is retrieved using magnetically-activated cell sorting, and antigen-specific Fab / phage are el...
example 2
Genetic and Immunological Properties of Phage-Displayed Human Anti-Rh(D) Antibodies
[0172] Clinically, the human Rh(D) antigen is the most important red blood cell (RBC) membrane protein in transfusion medicine. The autoimmune response against Rh(O) produces high affinity IgG antibodies which cause hemolytic transfusion reactions and hemolytic disease of the newborn (HDN). The prophylactic use of Rh(D)-immune globulin in pregnant Rh(D)-negative women has been a major advance in the prevention of HDN, yet the mechanism by which the drug exerts its immune modulatory effect is not well understood.
[0173] Monoclonal antibodies derived from the B cells of Rh(D)-immune globulin donors have defined several dozen Rh(D) epitopes (Scott, 1996, Transfus. Clin. Biol. 3:333). Paradoxically, the Rh(D) antigen, a circa 30 kD transmembrane. protein, has minimal extracellular mass and presents a very limited surface area for epitope expression. Because molecular cloning of a large repertoire of anti...
example 3
Isolation of Anti-Rh(D) Monoclonal Antibodies to Conventional and Novel Epitopes Using a Heavy Chain / Light Chain Shuffling Approach
[0357] In view of the results obtained in Examples 1 and 2 herein, heavy and light chains of antibodies of various Rh(D) epitope specificities were randomly recombined in order to generate anti-Rh(D) antibodies having additional patterns of reactivity with Rh(D) variant cells. Using this approach, plasmid DNA obtained from the Fab / phage display libraries described in panning rounds 2 and 3 of Example 1 was randomly recombined to generate a “shuffled” Fab / phage display library. When the Rh(D) specificity of antibodies of this “shuffled” library was determine, it was found that many of these antibodies exhibited novel epitope specificity. Significantly, antibody clones having novel Rh(D) epitope specificity were identified, including clones which bind to wild type and certain partial D type red blood cells but which do not bind to D category III red blood...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mole fraction | aaaaa | aaaaa |
| mole fraction | aaaaa | aaaaa |
| mole fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com