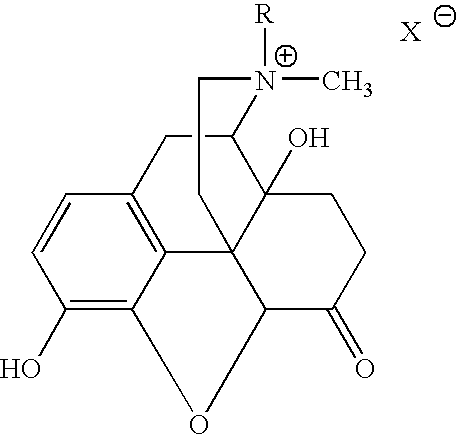
Use of methylnaltrexone and related compounds for treatment of gastrointestinal dysfunction induced by endogenous opioids
a technology of endogenous opioids and methylnaltrexone, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, biocides, etc., can solve the problems of pruritus, or itching, and the use of opioids is associated with a number of undesirable side effects, and may be very sever
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037]Ten patients were treated with morphine sulfate administered directly to the central nervous system or intravenously. The morphine sulfate was administered at 0.1 mg / kg body weight. The patients in the study had been treated for pain resulting from surgery. All the patients exhibited pruritus as a side effect of the morphine sulfate administration. Subsequent to the onset of the pruritus, methylnaltrexone, at a dosage of 0.3 mg / kg of body weight was administered intravenously as a saline solution containing methylnaltrexone in a concentration of 5 mg / ml to each of the patients. Eighty percent of the 10 patients exhibited relief from the pruritus sixty minutes after receiving methylnaltrexone.
[0038]In a control group, 8 patients were treated with morphine sulfate administered directly to the central nervous system or intravenously. The morphine sulfate was administered at 0.1 mg / kg body weight. The patients in the study had been treated for pain resulting from surgery. All the ...
example 2
Efficacy of Enteric Coating of Methylnaltrexone
[0040]Morphine (0.05) mg / kg intravenous) was administered to three volunteers after the oral administration of placebo, methylnaltrexone (6.4 mg / kg) in a gelatin capsule (which dissolves readily in the stomach), or methylnaltrexone after enteric coating (12.8 mg / kg of substance to yield a mass of 6.4 mg / kg methylnaltrexone incorporated) which has decreased release and absorption in the stomach. Oral-cecal transit time was measured using the lactulose-hydrogen breath test. Plasma levels of methylnaltrexone were measured and after the enteric coated preparation were lower. In each subject morphine alone increased the oral-cecal transit time by 20-70 minutes, methylnaltrexone blocked this effect, and enteric coated methylnaltrexone blocked the effect to a similar or greater extent than the uncoated methylnaltrexone.
example 3
Enhancement of Enteric Feeding
[0041]Two patients receiving morphine (375 mg / day and 18 mg / day) and receiving enteric tube feedings of 200 ml every four (4) hours were studied. The first patient had residual stomach contents of 50 cc to 100 cc, or 22.0-58.8% of administered feedings measured every 4 hours during a 24 hour control period. Prior to drug administration the residual volume had increased to 260 cc or >100% of previous feeding volume. Methylnaltrexone, 0.45 mg / kg, was administered intravenously every 4 hours for 24 hours, after the control period. After the first dose (4 hours) of MNTX, the residual was 150 cc or 58% of the previous bolus feed, after the 3rd dose (12 hours) the residual was 75 cc or 30% of the previous feed, after the 5th dose (20 hours) the residual was 22 cc or 13% of the previous feed and after the 6th and final dose (24 hours) the residual was 8 cc or 5.5% of previous feed. The follow-up residual sampling after the final drug-tube feed interval had inc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| lipid soluble | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com