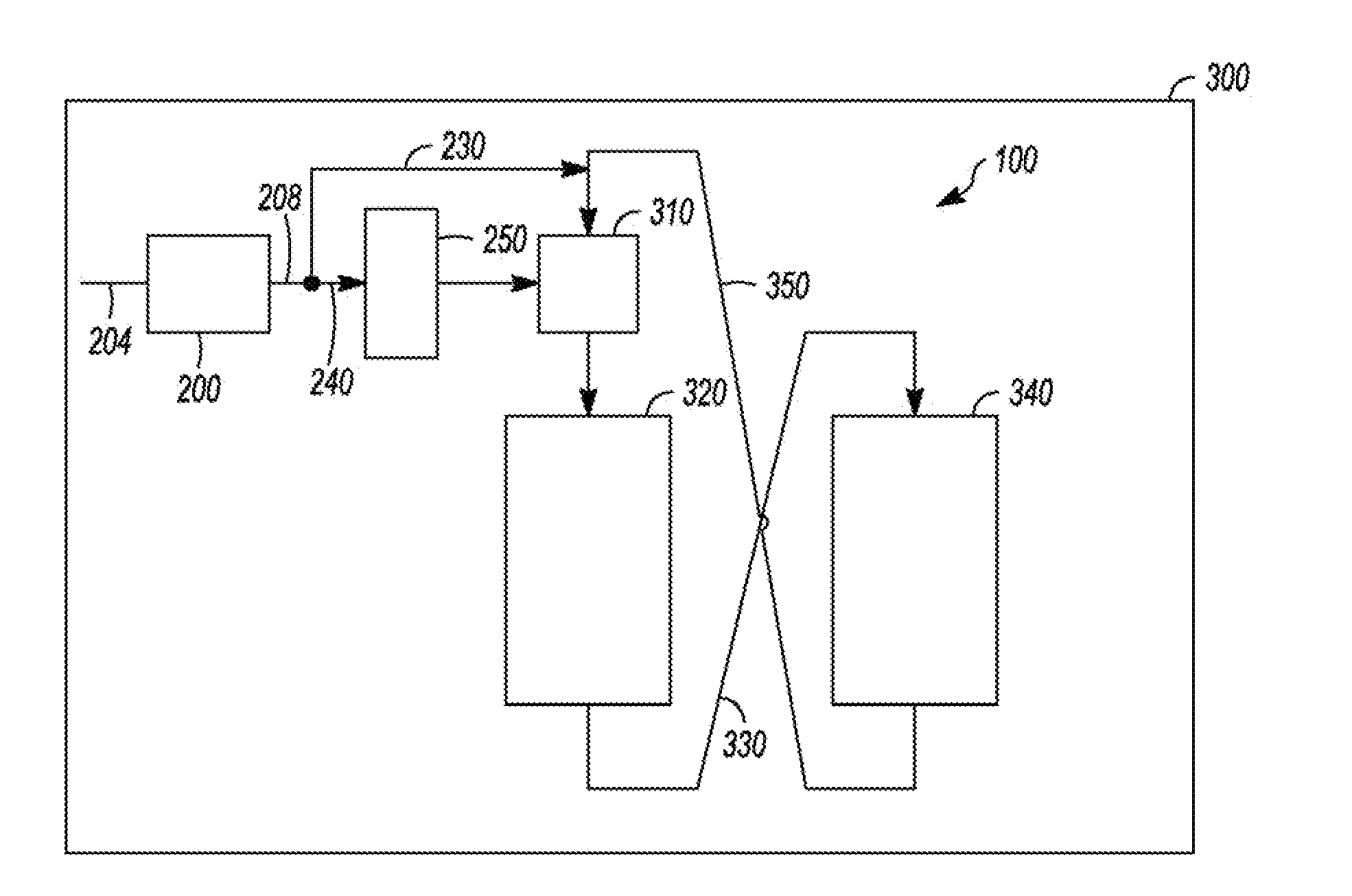
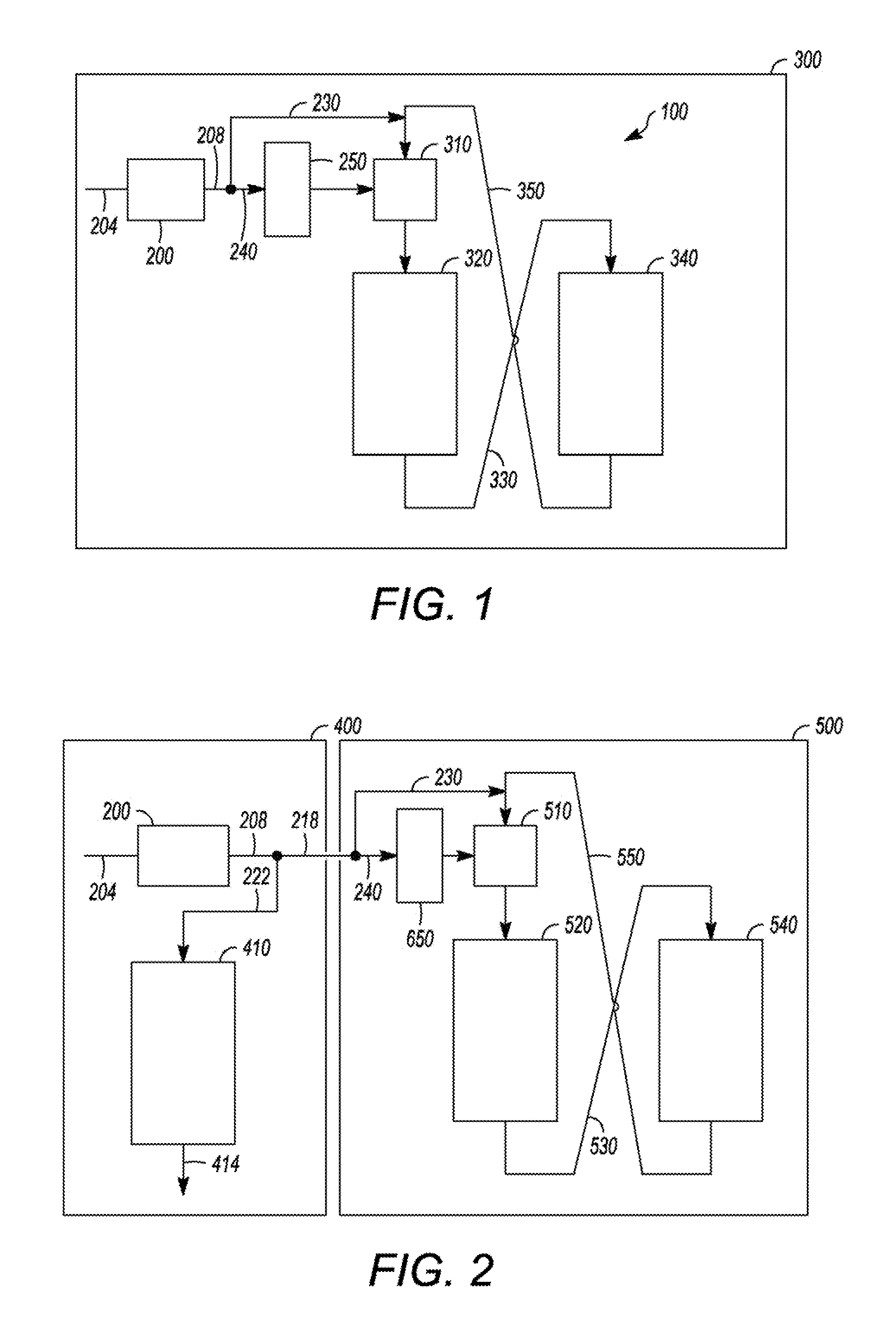
Apparatus and Process for Removal of Carbon Monoxide
a carbon monoxide and apparatus technology, applied in the direction of catalyst regeneration/reactivation, physical/chemical process catalysts, separation processes, etc., can solve the problems of reducing the selectivity of desired products, reducing capacity, and increasing coking, so as to reduce the level of carbon monoxide, reduce the cost of catalyst coking, and improve the effect of operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0030]Two catalysts are prepared with a spherical alumina support. The first catalyst has a final composition of 0.25%, by weight, platinum (Pt) and 0.30%, by weight, tin (Sn) (catalyst A) while a second catalyst has a final composition of 0.30%, by weight, Pt and 0.30%, by weight, Sn (catalyst B). Each catalyst is oxychlorinated to disperse the platinum and achieve a chloride level of about 0.9-about 1%, by weight, chloride (Cl) on the catalyst.
[0031]Each catalyst is then exposed to different reduction conditions in a reforming pilot plant using naphtha feed of 55.5% paraffins, 31.7% naphthenes, and 12.8% aromatics (all percents by weight) at a hydrogen:hydrocarbon mole ratio of 2, a liquid hourly space velocity (LHSV) of 1.7 hr−1, and a pressure of 620 kPa. Catalyst activity is determined by the temperature needed to maintain a target octane. Yields are calculated based on on-line gas and liquid effluent chromatography analysis. Runs are equal in length of time and spent catalyst ...
example 2
[0032]Similar experiments are conducted as in Example 1 using a commercially manufactured continuous catalyst regeneration catalyst (catalyst C) containing 0.25% Pt, 0.3% Sn, and 0.94% Cl (all percentages by weight). Catalyst C is split into two portions for reduction at temperatures of 399° C. and 566° C. in the presence of 0 vppm carbon monoxide. Results are depicted in Table 2.
TABLE 2Yield and Activity Results of 56 m3 of Naphtha Feed Per m3 of CatalystH2 Reduction ConditionsPilot Plant ResultsClTimeTemp.COC5+Activity Temp.Average CarbonCatalyst(wt. %)(hour)(° C.)(vppm)(wt. %)(° C.)(g / 100 cc)Delta %C0.902399086.25192.44BaseC0.782566086.35192.03−16.8%
[0033]Results indicate that higher reduction temperatures produce about 17% less coke for catalyst C. The C5+ yields and activity remain relatively constant.
example 3
[0034]Further experiments with catalyst A and B are conducted for up to 10 hours, in the presence of 0 vppm of carbon monoxide with samples analyzed at 2, 4 and 10 hours. The data at 4 hours is from Table 1. Results are depicted in Table 3.
TABLE 3Yield and Activity Results of 56 m3 of Naphtha Feed Per m3 of CatalystH2 Reduction ConditionsPilot Plant ResultsClTimeTemp.COC5+Activity Temp.Average CarbonCatalyst(wt. %)(hour)(° C.)(vppm)(wt. %)(° C.)(g / 100 cc)Delta %A0.9845651086.65171.74BaseA0.942565086.35161.42−18.4%A0.8710565086.15181.34−23.0%B0.9945651086.85182.15BaseB0.952565086.55171.77−17.7%B0.8710565086.85181.56−27.4%
[0035]As depicted, extended reduction time in substantially carbon monoxide free hydrogen gas results in further coke reduction ranging from about 23-about 27% reduction, as compared to the base condition of 4 hours, as depicted in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com