Fixation device and method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
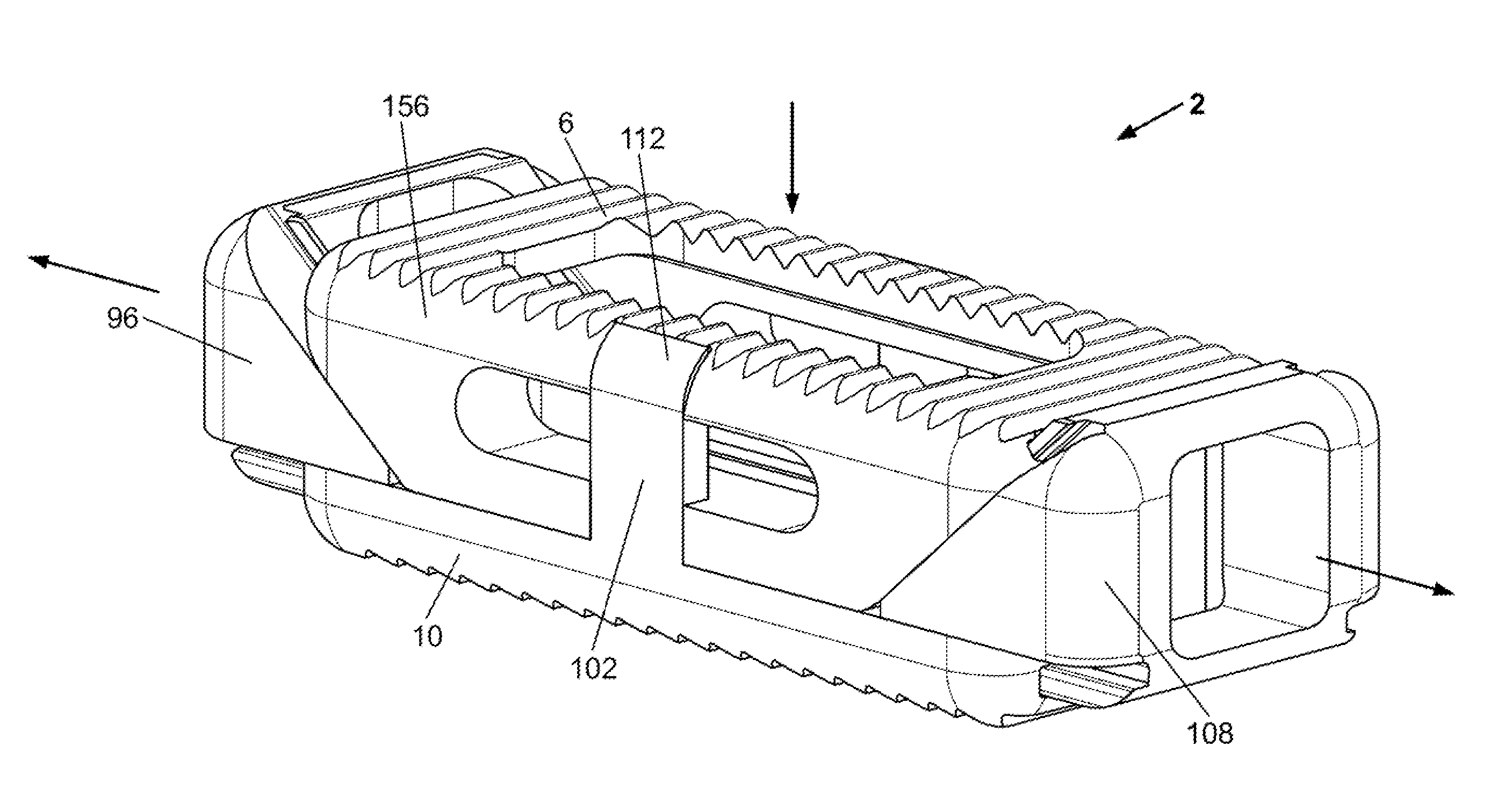
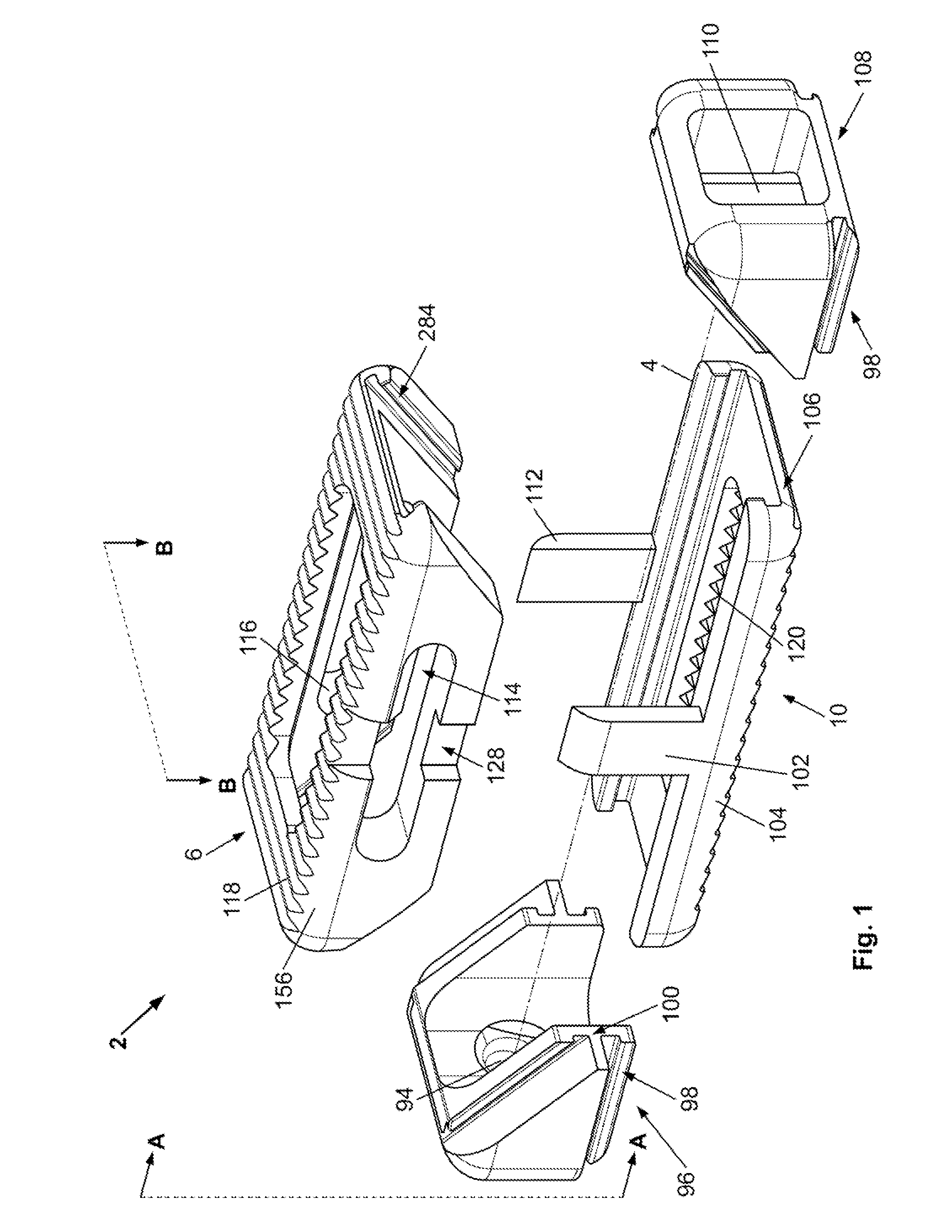
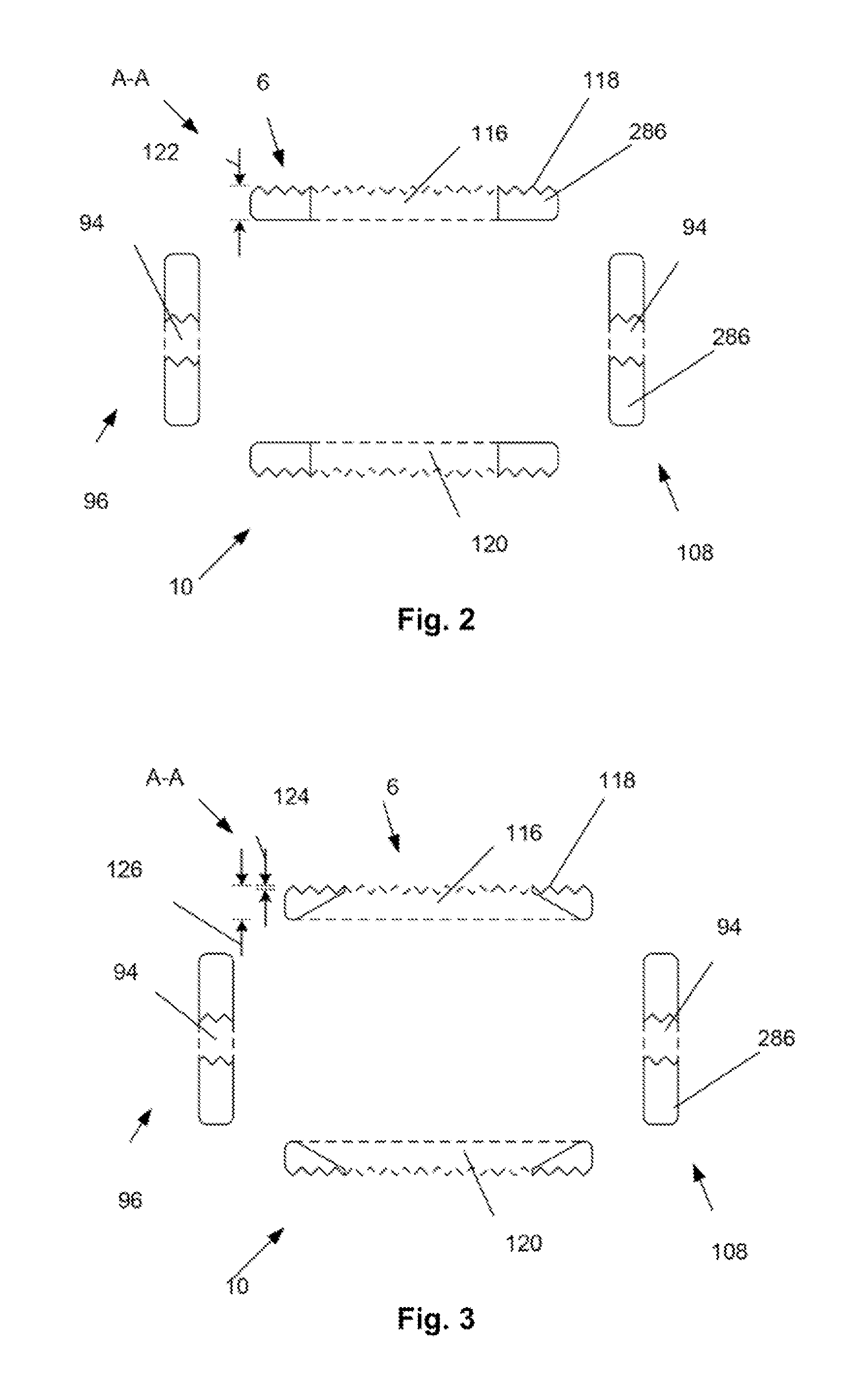
[0037]A device 2 is disclosed that can be inserted into a target site 264 with the device 2 in a compressed or contracted (i.e., small) configuration. Once positioned in the deployment site, the device 2 can be transformed into an expanded (i.e., larger, bigger) configuration. The device 2 can be inserted and expanded in orthopedic target sites 264 for fixation and / or support. For example, the device 2 can be inserted and expanded over a guidewire between adjacent vertebral bodies.
[0038]FIG. 1 illustrates that the device 2 can have a first longitudinal end and a second longitudinal end along a longitudinal axis 4. The device 2 can have a bottom or plate 286 (bottom and base plate are used interchangeably) and a top plate 6. The base 138 or bottom plate 10 and top plate 6 can be or have plates 286, panels, struts 216 (e.g., legs), ports, cells 88, and combinations thereof. The base plate 10 and top plate 6 can be configured to be slidably attachable to the other. For example, the bas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com