Recombinant probiotic bacteria for the prevention and treatment of inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS)
a probiotic and inflammatory bowel disease technology, applied in the field of gut inflammatory diseases, can solve the problems of limited efficacy, inability to fully understand the mechanisms involved in the pathogenesis of ibd, and decreased disease activity in crohn's disease patients treated with il-10 recombinant i>l. lactis /i>, so as to improve the efficacy of treatment and ensure the effect of safety and better efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Material & Methods
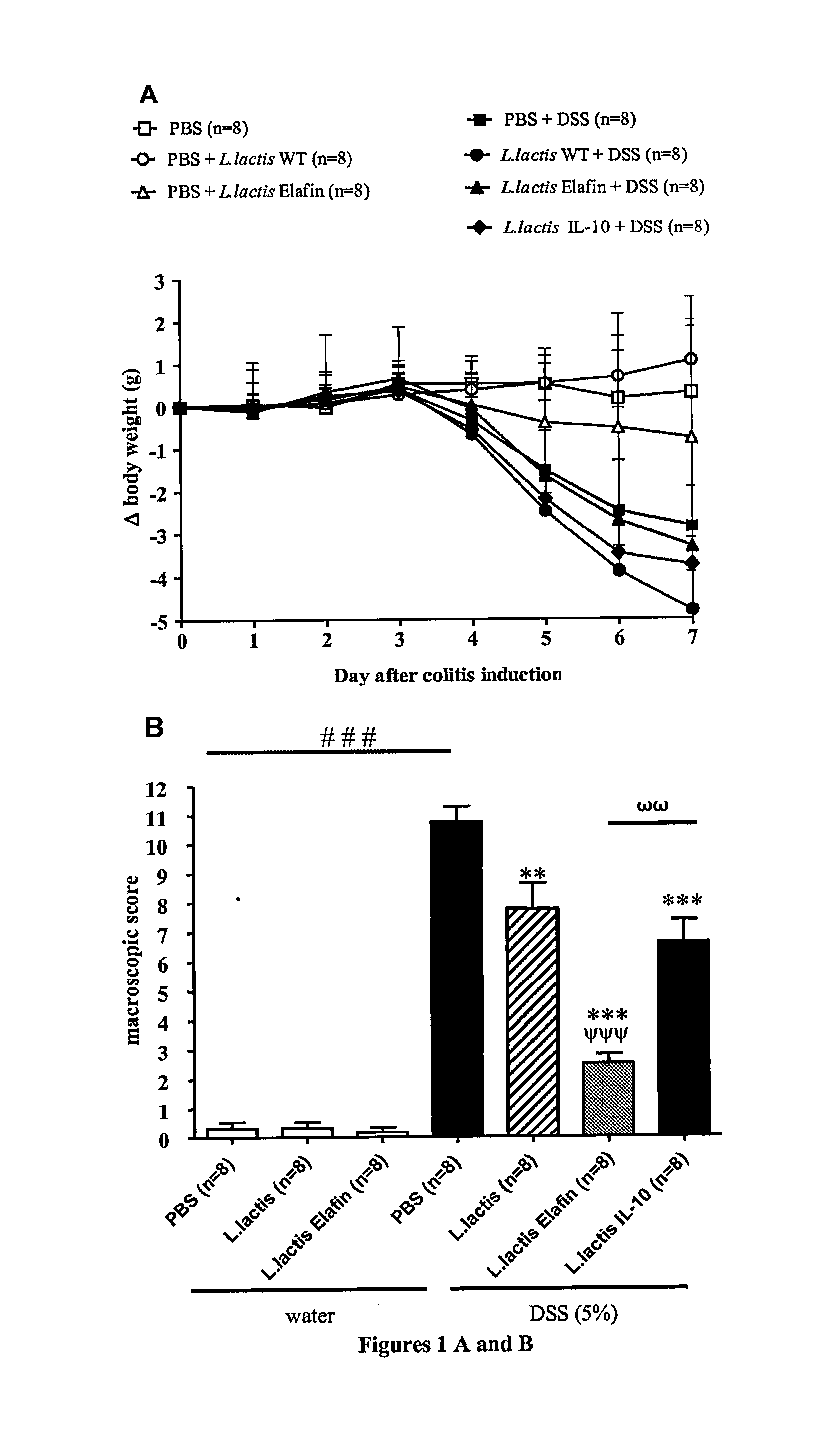
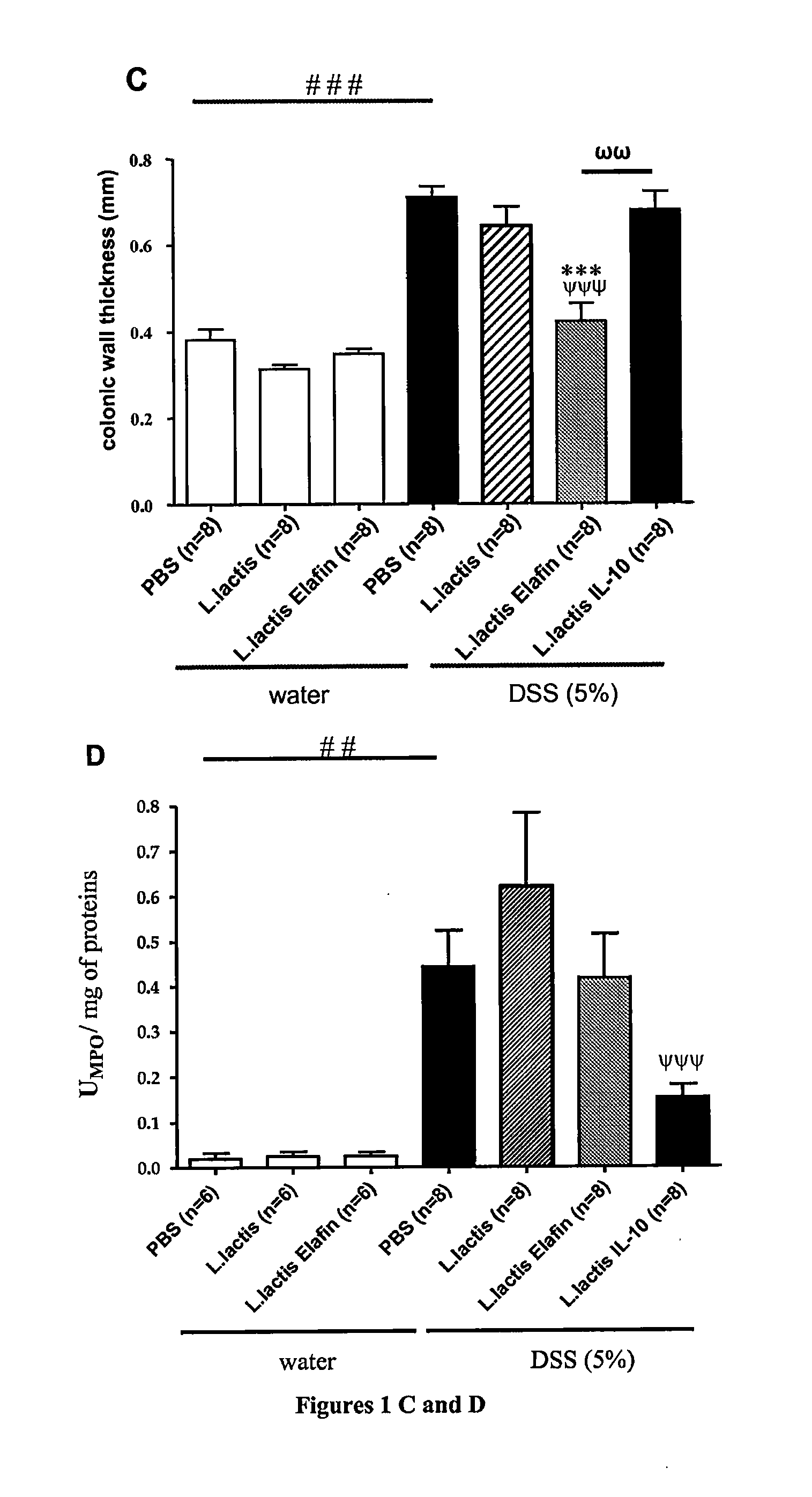
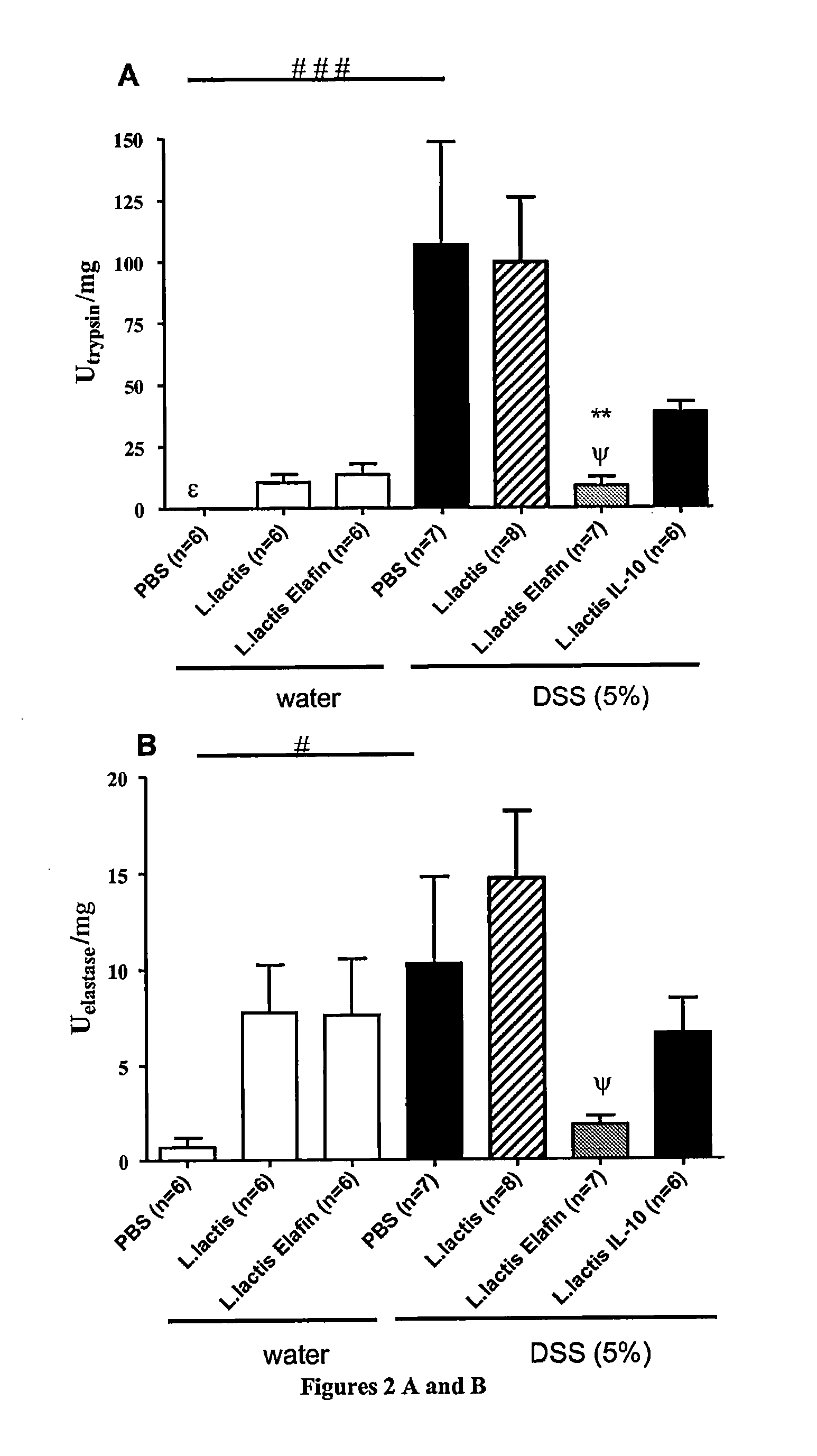
[0084]Cloning of Elafin in recombinant lactic acid bacteria (Lactococcus lactis and Lactobacillus casei)
Cloning and Expression of Elafin in Lactic Acid Bacteria
[0085]Gene coding for elafin was PCR amplified from plasmid DK6-elafin. Sequences of primers used were: 5′ forward Elafin (CCAATGCATCAGCAGCTGTCACGGG AGTTCC) (SEQ ID No. 1) and 3′ reverse-Elafin (GGACTAGTCCTCACTGGGGAACGAAACA GGCC) (SEQ ID No. 2). Primers were designed to eliminate first codons of elafin region encoding for signal peptide (SP) and was replaced by the SP of Usp45 protein (PSUsp45), the main secreted protein from L. lactis. To that aim, PCR product was digested, purified, and cloned in pSEC, a L. lactis secretion vector. In the resulting plasmid pSEC:elafin, elafin is fused in frame with a DNA fragment encoding for RBS and PSUsp45. Expression of the cassette is controlled by the inducible promoter PnisA, the activity of which depends upon the concentration of nisin used. This plasmid was then in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com