High concentration antibody-containing liquid formulation
a liquid formulation, high concentration technology, applied in the direction of antibody medical ingredients, immunological disorders, drug compositions, etc., can solve the problems of antibody molecules being lost, bioactivity is lost, and there is not yet a technology sufficient to prevent dimerization and deamidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
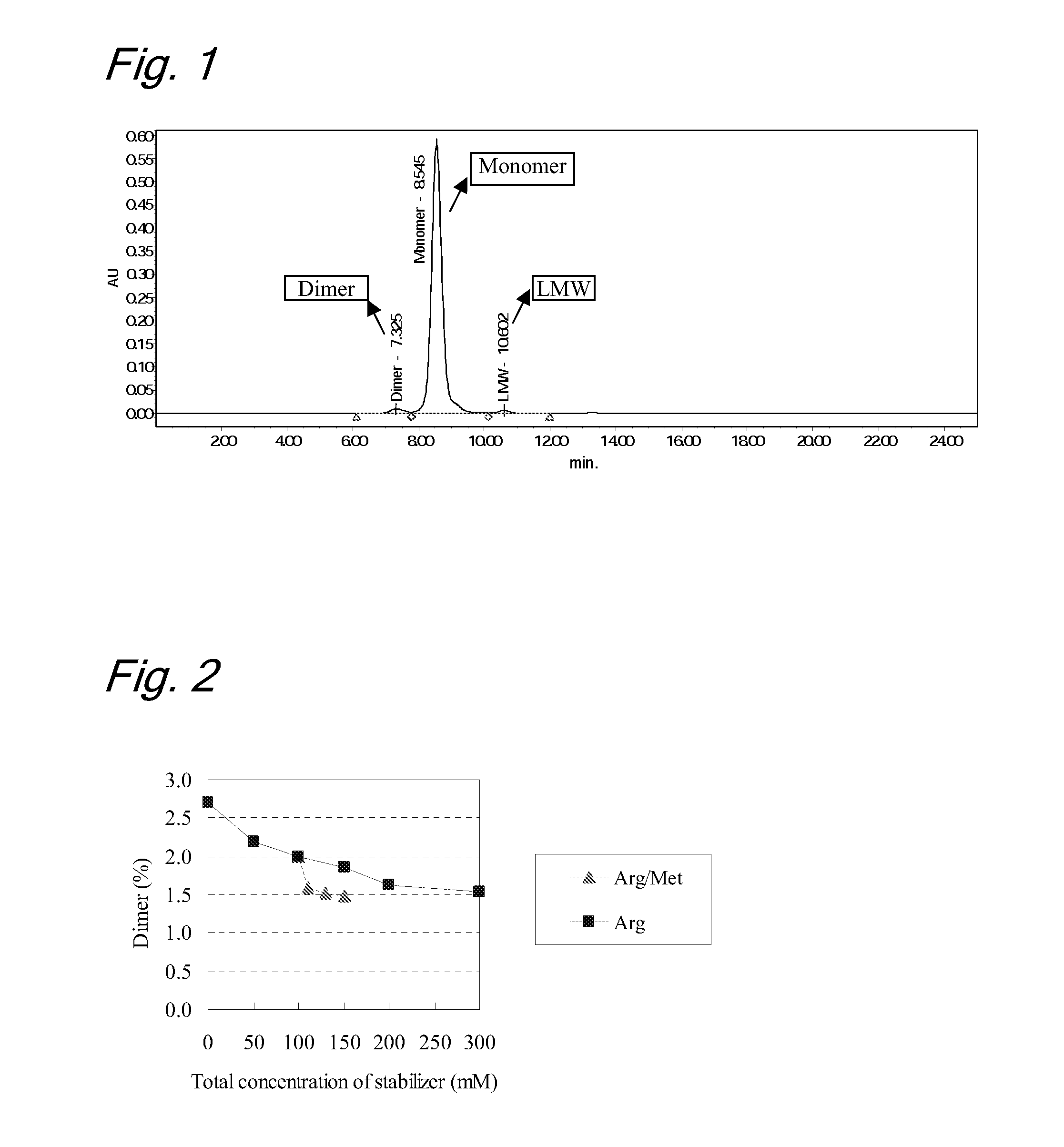
[0094]Stabilizing Effects by Combination of Arginine and Methionine
[0095]Liquid formulations containing anti-IL-6 receptor humanized antibody were evaluated for an influence on stabilization of the formulations obtained by use of a combination of arginine and methionine.
[0096]In this study, to evaluate the effects by the combination of arginine and methionine, evaluation samples numbered A1 to A9 were prepared. Prescriptions for the evaluation samples were as follows:
TABLE 1-1[Prescriptions]SampleAntibodyArgMetPolysorbate 80Histidine bufferNo.mg / mLmMmMmg / mLmMpHA1180——0.5206.0A2180 50—0.5206.0A3180100—0.5206.0A4180150—0.5206.0A5180200—0.5206.0A6180300—0.5206.0A7180100100.5206.0A8180100300.5206.0A9180100500.5206.0
[0097]To evaluate stability of the liquid formulations, each sample was subjected to a heat acceleration test (stored at 40° C. for 3 months and at 25° C. for 6 months, respectively). The purity of the antibody before and after the heat acceleration test was evaluated by gel ...
example 2
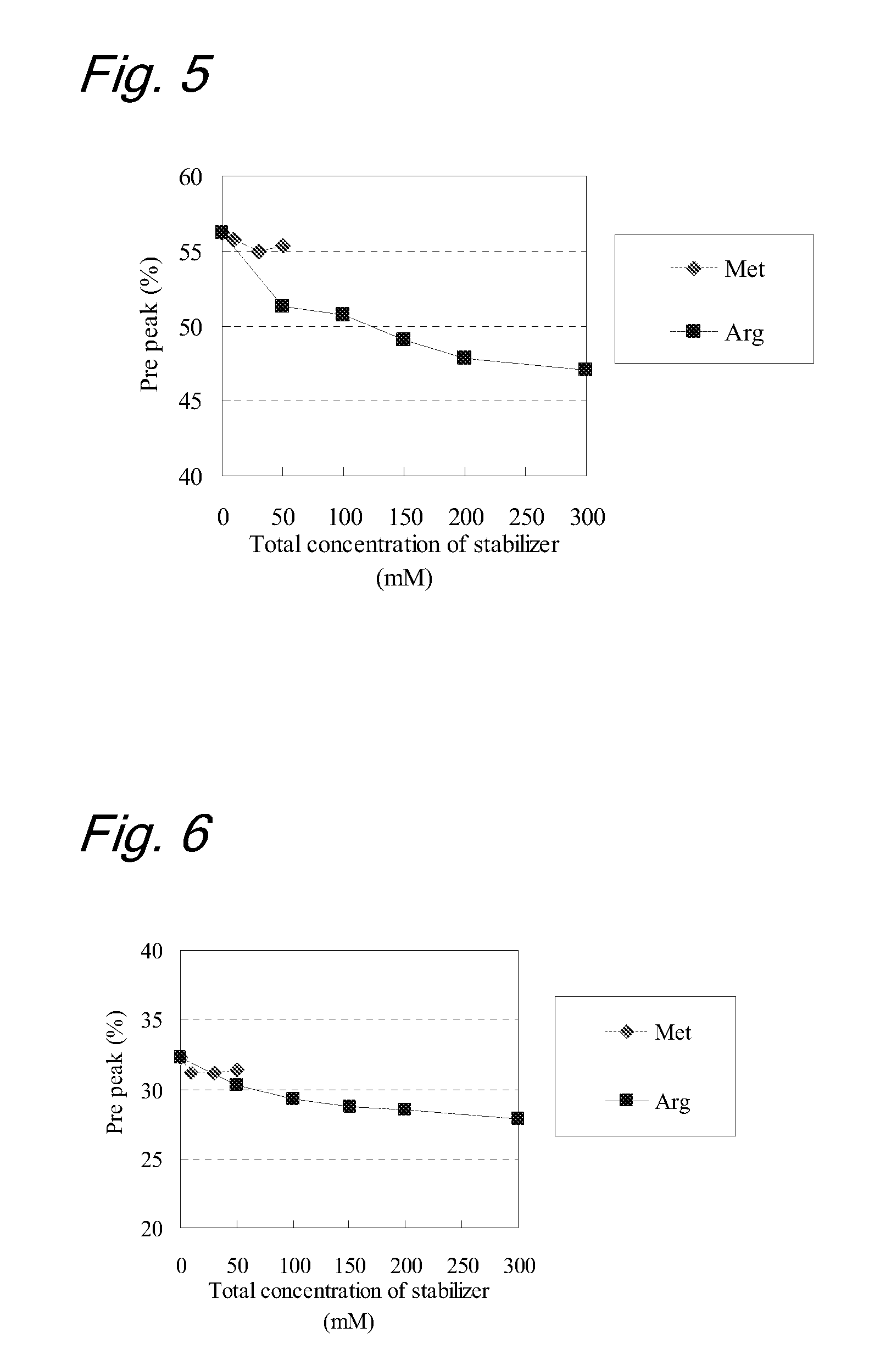
[0106]Inhibitory Effect by Arginine against Deamidation
[0107]Liquid formulations containing and-IL-6 receptor humanized antibody were evaluated for influence on the deamidation by arginine.
[0108]In this study, evaluation samples numbered A10 to A15 and numbered A16 to A18, containing different amounts of arginine and methionine, respectively, were prepared.
[0109]Prescriptions for the evaluation samples were as follows:
TABLE 2-1[Prescriptions]SampleAntibodyArgMetPolysorbate 80Histidine bufferNo.mg / mLmMmMmg / mLmMpHA10180——0.5206.0A11180 50—0.5206.0A12180100—0.5206.0A13180150—0.5206.0A14180200—0.5206.0A15180300—0.5206.0A16180—100.5206.0A17180—300.5206.0A18180—500.5206.0
[0110]To evaluate the stability of the liquid formulations, each sample was subjected to a heat acceleration test (stored at 40° C. for 3 months and at 25° C. for 6 months, respectively). The purities of the antibody before and after the heat acceleration test were evaluated by ion-exchange chromatography (IEC). The analy...
example 3
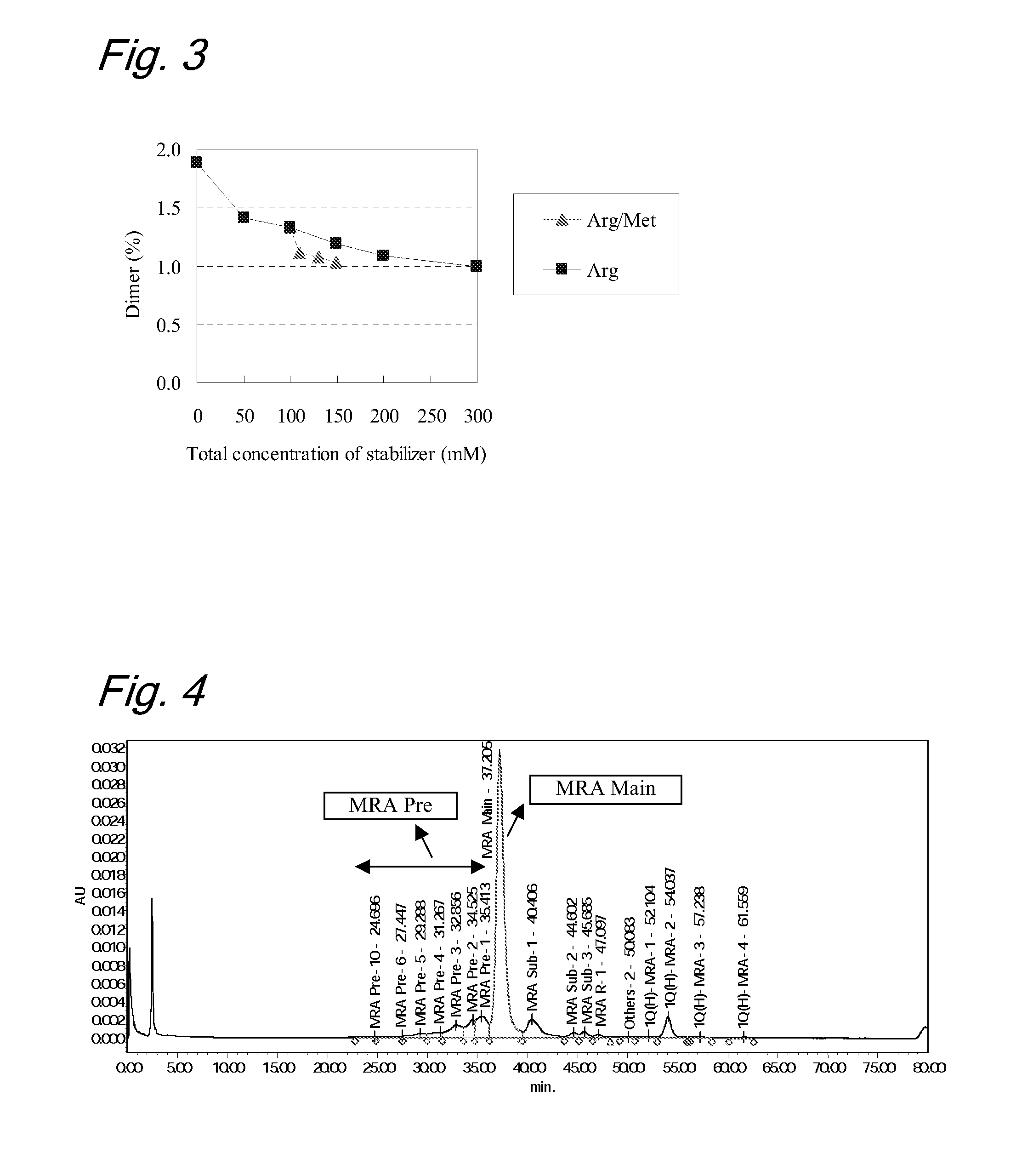
[0119]Stabilizing Effects by Combination of Arginine and Methionine (2)
[0120]As in Example 1, liquid formulations containing receptor humanized antibody were evaluated for influence on stabilization of the formulations obtained by use of a combination of arginine and methionine.
[0121]In this study, to evaluate effects of the combination of arginine and evaluation samples numbered A19 to A27 were prepared. Prescriptions for the evaluation samples were as follows:
TABLE 3-1[Prescriptions]SampleAntibodyArgMetPolysorbate 80Histidine bufferNo.mg / mLmMmMmg / mLmMpHA19180——0.5206.0A20180 50—0.5206.0A21180100—0.5206.0A22180150—0.5206.0A23180200—0.5206.0A24180300—0.5206.0A25180100100.5206.0A26180100300.5206.0A27180100500.5206.0
[0122]To evaluate the stability of the liquid formulations, each sample was subjected to a light acceleration test (total illuminance 1,200,000 lux and total near-ultraviolet radiation energy: 200 W·h / m2). The purities of the antibody before and after the light acceleratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com