Implanted cardiac device for defibrillation
a cardioverter and implantable technology, applied in the field of conventional implantable cardioverterdefibrillators, can solve the problems of battery performance degradation, need for battery replacement, and unnecessary removal of icd components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
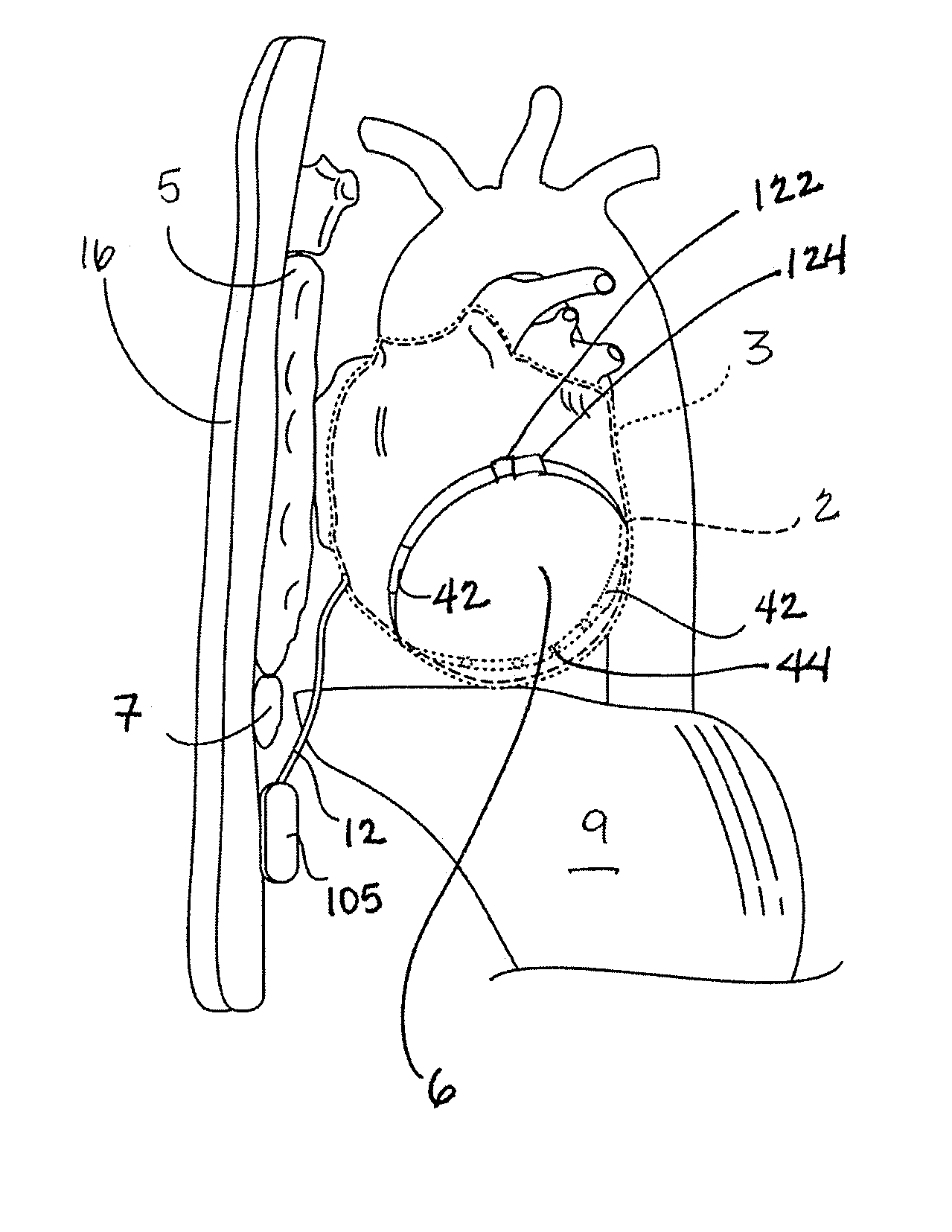
[0042]According to Rahul Mehra, PhD and Paul DeGroot, MS, in an article entitled, “Where are we, and where are we heading in the device management of ventricular tachycardia / ventricular fibrillation?” there are several challenges for increasing the acceptance of ICD therapy. Among the challenges is to improve the technology, facilitating implant and follow up from the physician's perspective and lowering morbidity from the patient's perspective. The same article points out that patients want ICDs that are smaller, result in fewer shocks, have less pain associated with defibrillation, require minimal follow-up, and last a long time so that the devices to not require replacement (Heart Rhythm, Volume 4, No. 1, January 2007). As will become clear in the detailed description that follows, embodiments of the ICD system and components of the present invention address these challenges and patient desires.
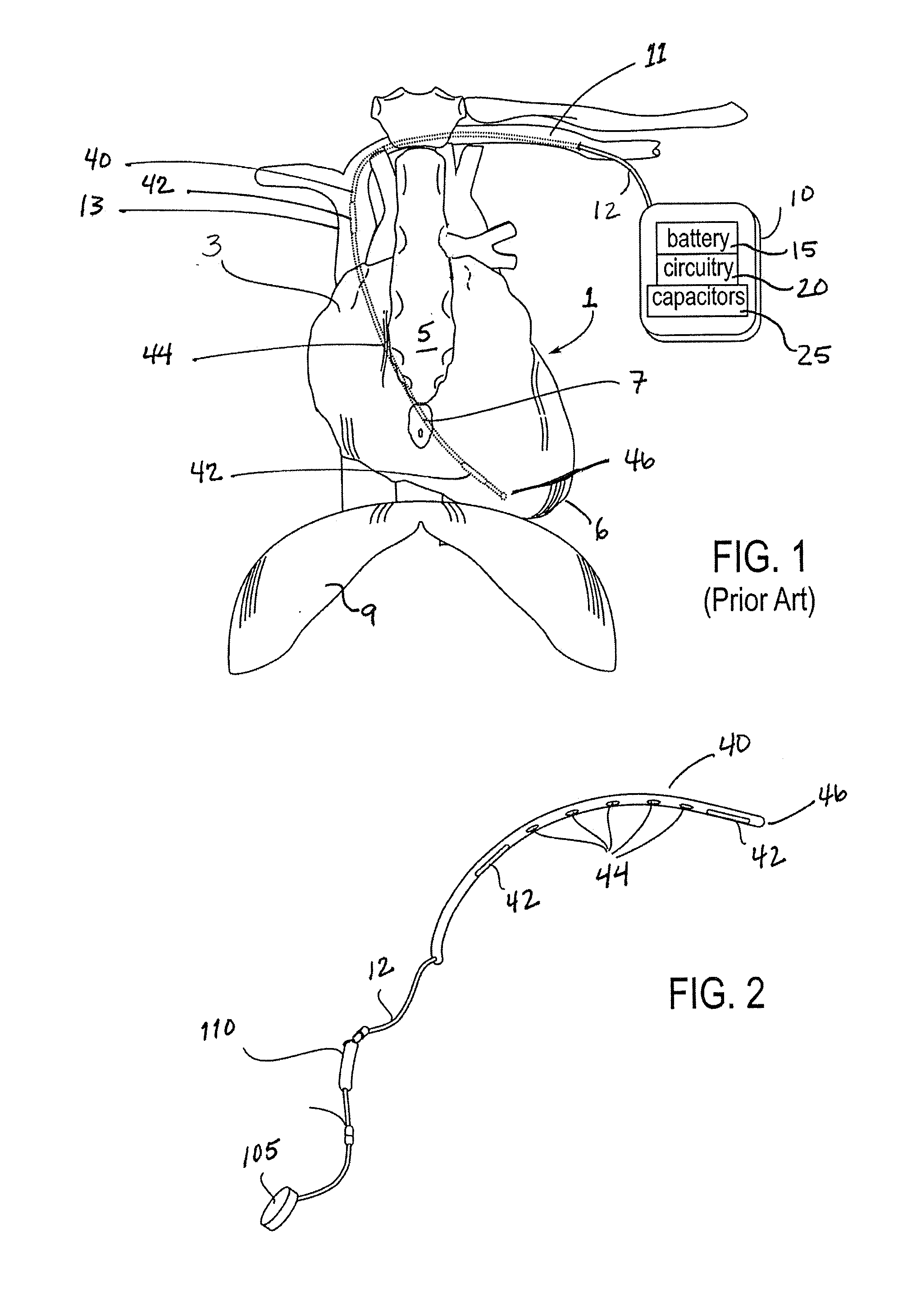
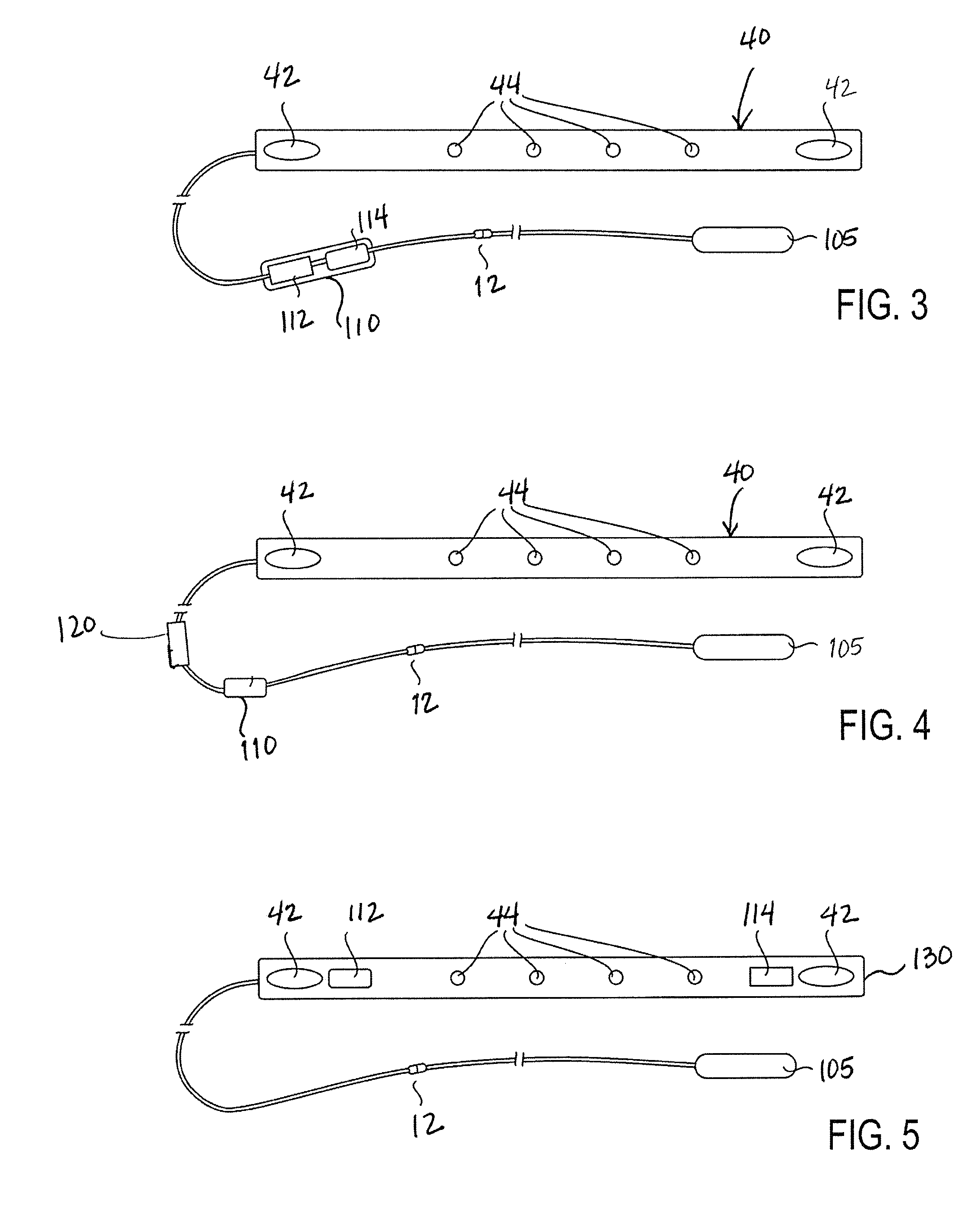
[0043]FIG. 2 illustrates an implantable medical device for delivering electrical cardi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com