Methods for Treating Cancer Using Pyrimidine and Pyridine Compounds with BTK Inhibitory Activity
a technology which is applied in the field of pyrimidine and pyridine compounds, can solve the problems of difficult to develop a reversible inhibitor that selectively inhibits a desired (la, target) kinase, difficult to maintain inhibition over extended periods of time, and high dosages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Mosaic Spot Assay (CLL Patient Samples)
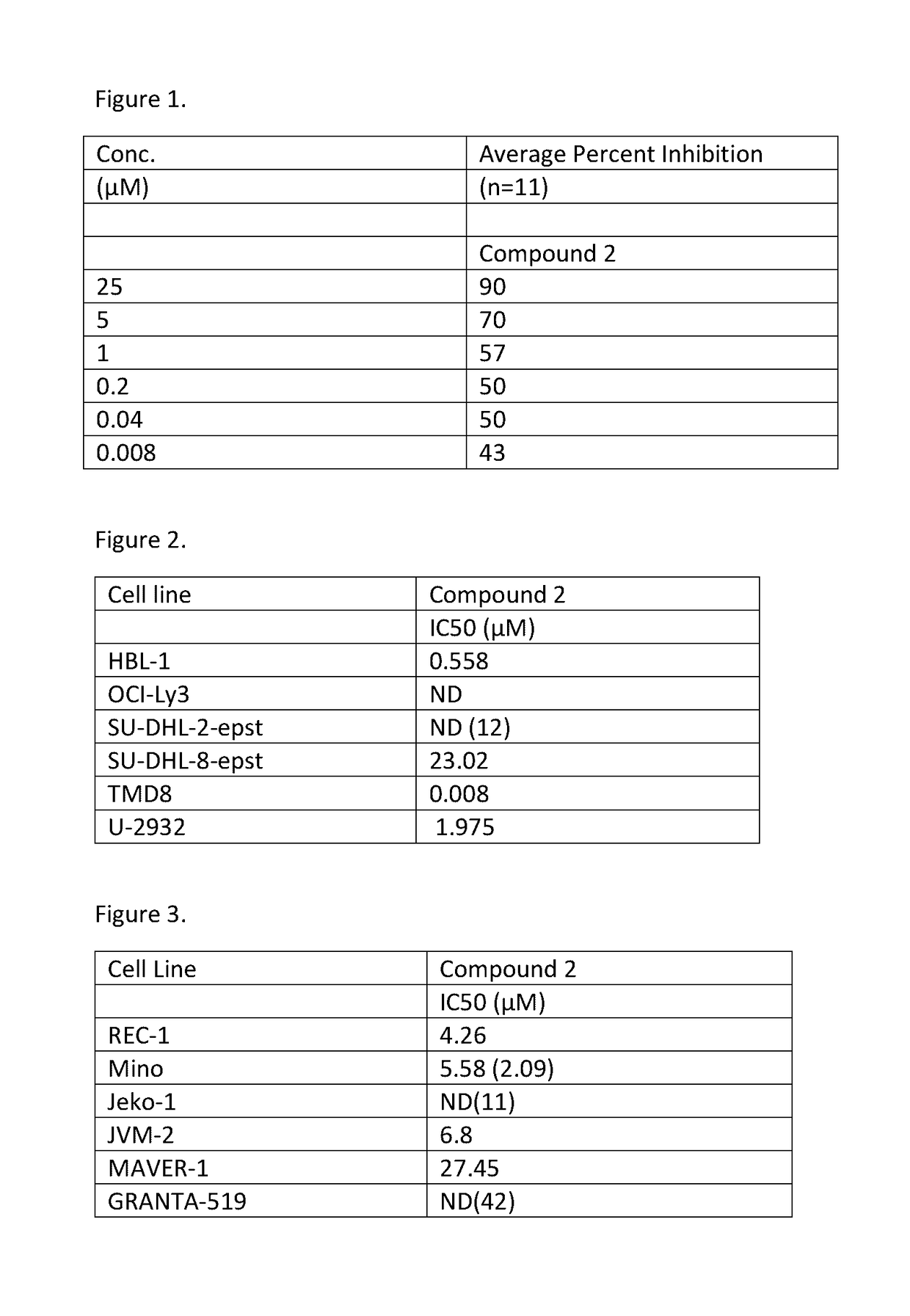
[0175]The Mosaic Spot Assay was conducted in accordance with Mosaic Laboratories' SOPs. The CLL blood and bone marrow mononuclear cells were evaluated for viability and cell count prior to plating in Mosaic Spot Media (X-Vivo 10 Media [Lonza, Fisher Scientific, Carlsbad, Calif.]+10% FBS [Life Technologies, Carlsbad, Calif.]) in a 96-well cytophobic plate in the presence of compounds and two controls: 1) a cytotoxic dose of cisplatin serving as a positive control; and 2) no drug treatment serving as a reference and negative control. Tumor cells were placed in a humidified 37° C. incubator with 5% CO2 for 4 days. At the end of the incubation, cells were removed and cytocentrifuged onto positive charged glass slides (Superfrost+, VWR, Radnor, Pa.). Slides were stained with Wright Giemsa in accordance with Mosaic Laboratories SOPs. Cell growth was measured by a hematopathologist. The results are provided in FIG. 1.
example 2
MCL / ABC-DLBCL Cell Lines
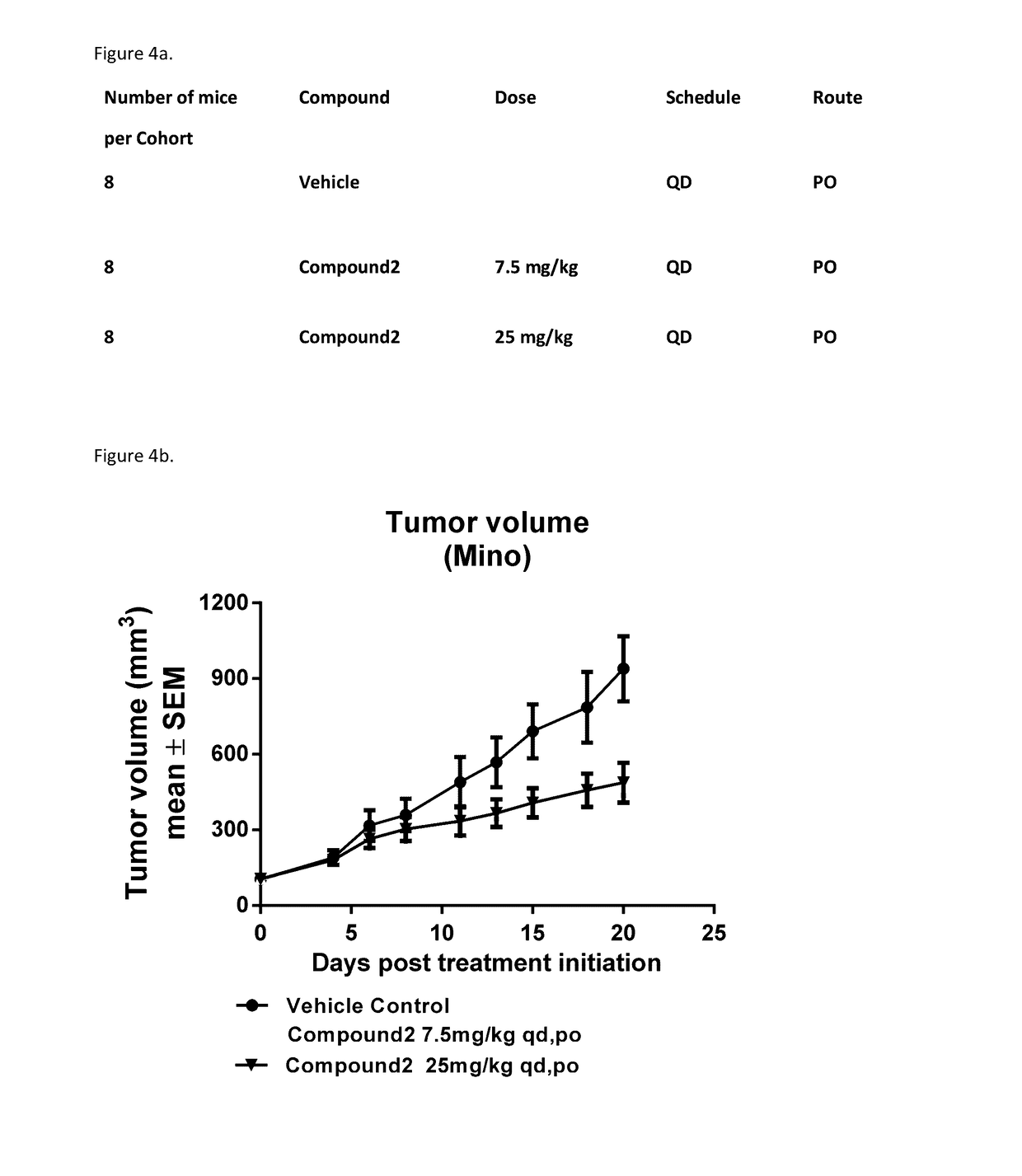
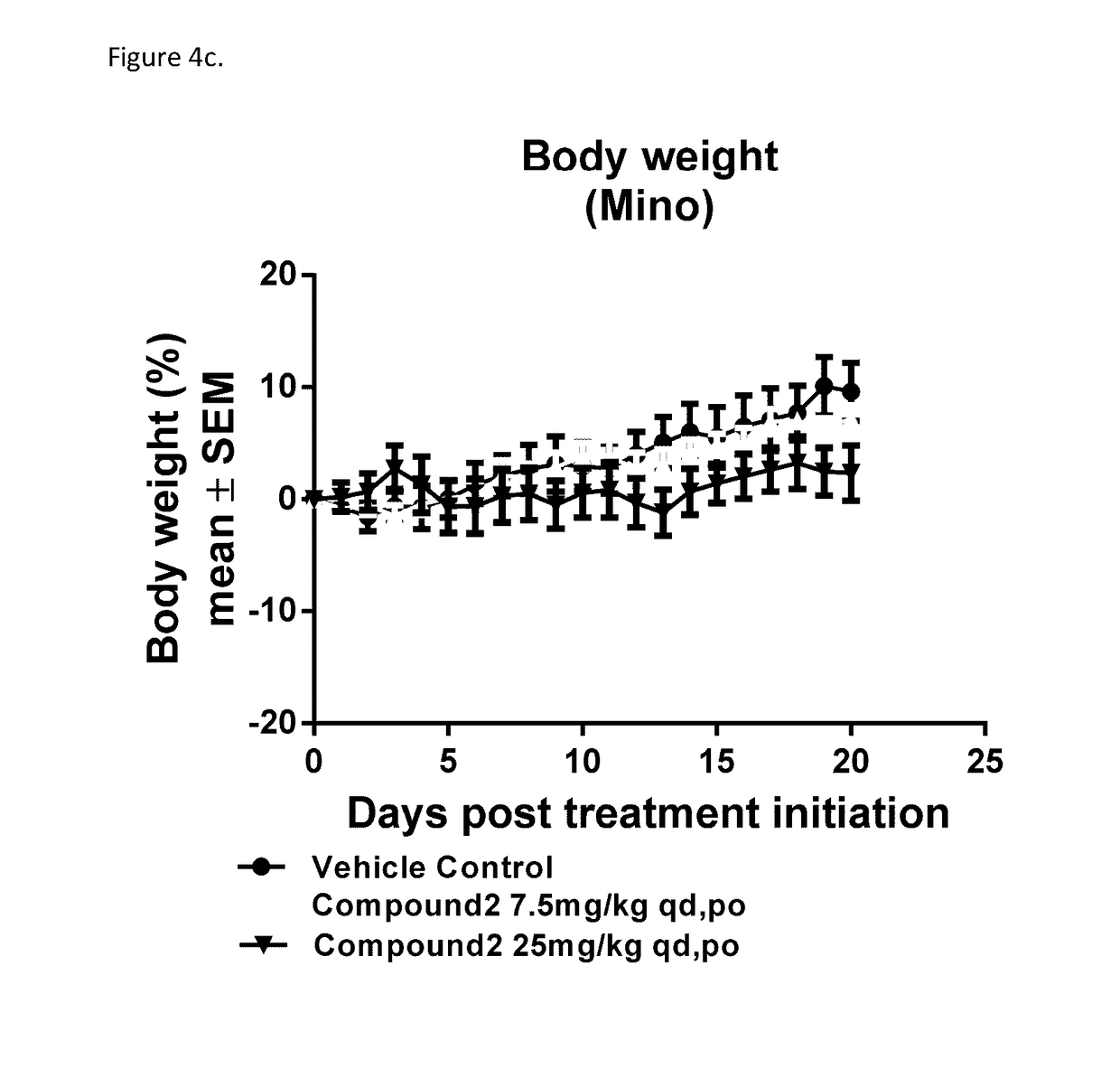
[0176]Cell lines that were preserved in liquid nitrogen were thawed and expanded in growth media containing full serum. Once cells reached expected doubling times, screening began. Cells were seeded in growth media in black 384-well tissue culture treated plates. Cells were equilibrated in assay plates via centrifugation and placed in incubators (attached to the Dosing Modules) at 37° C. for twenty-four hours before treatment. At the time of treatment, a set of assay plates (which do not receive treatment) were collected and ATP levels were measured by adding ATPLite (Perkin Elmer). These Tzero (T0) plates were read using ultra-sensitive luminescence on Envision plate readers. Assay plates were incubated with compound for seventy-two hours and were analyzed using ATPLite. All data points were collected via automated processes and were subject to quality control and analyzed using Horizon's proprietary software. Assay plates were accepted if they passed the fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Force | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com