Glycosylated yghj polypeptides from enterotoxigenic escherichia coli (ETEC)
a technology of enterotoxigenic escherichia coli and glycoprotein, which is applied in the field of glycoprotein-yghj polypeptides from, can solve the problems of significant negative health- and socio-economic impact of etec infection, complex discovery and characterization of glycoproteins, and poor ionization of peptides modified, and achieves the effect of improving the ionization efficiency of peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
BEMAP Method Results
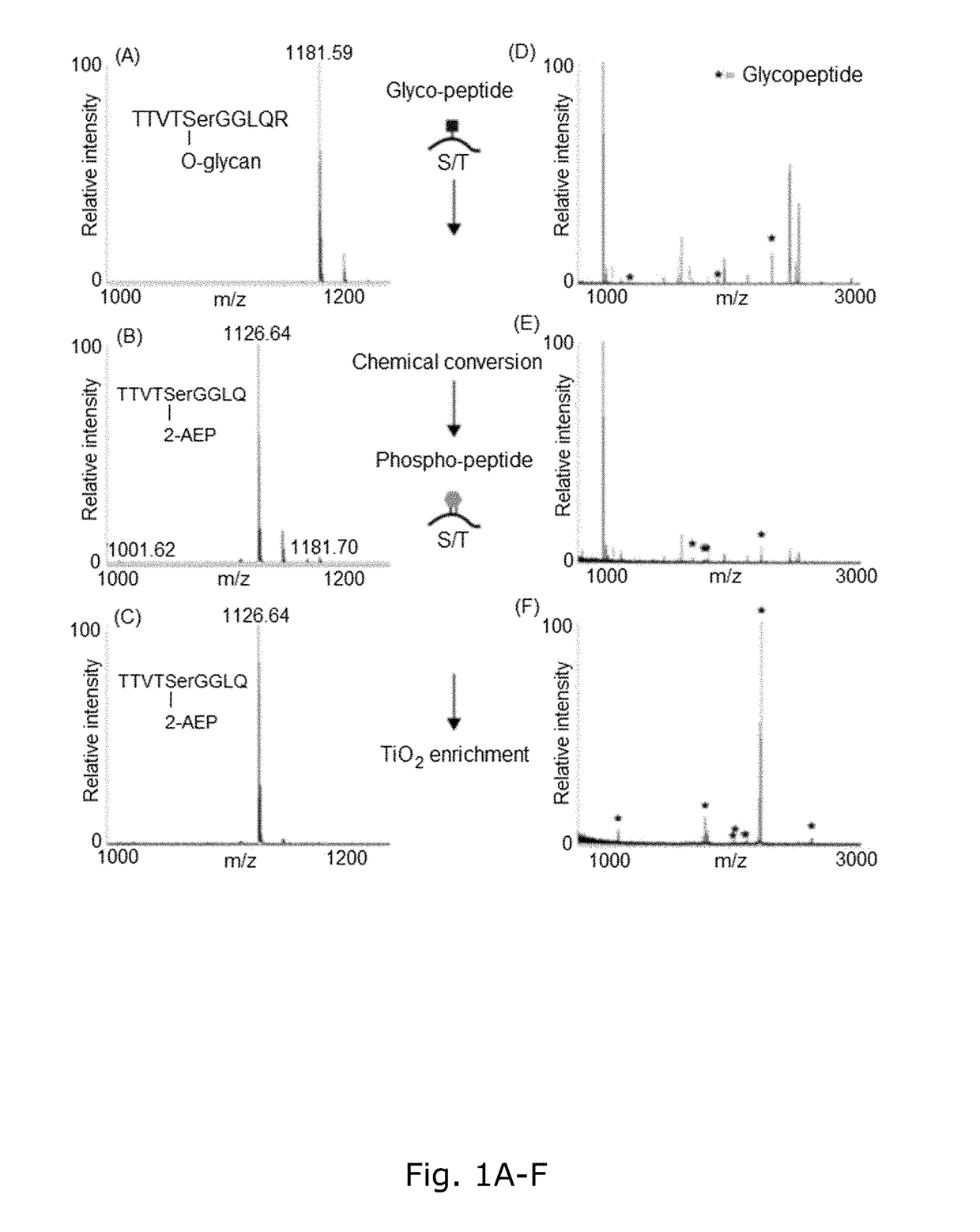
[0198]BEMAP relies on β-elimination of O-linked carbohydrate modifications, Michael addition of 2-Aminoethyl phosphonic acid (AEP) and TiO2 enrichment of phosphopeptides. Thus, BEMAP combines a firmly established in vitro chemical modification with a highly selective enrichment protocol (Thingholm et al., 2006) and the reactions take place in a single volume without the need for intermediate purification steps as described in the Experimental Procedures section.
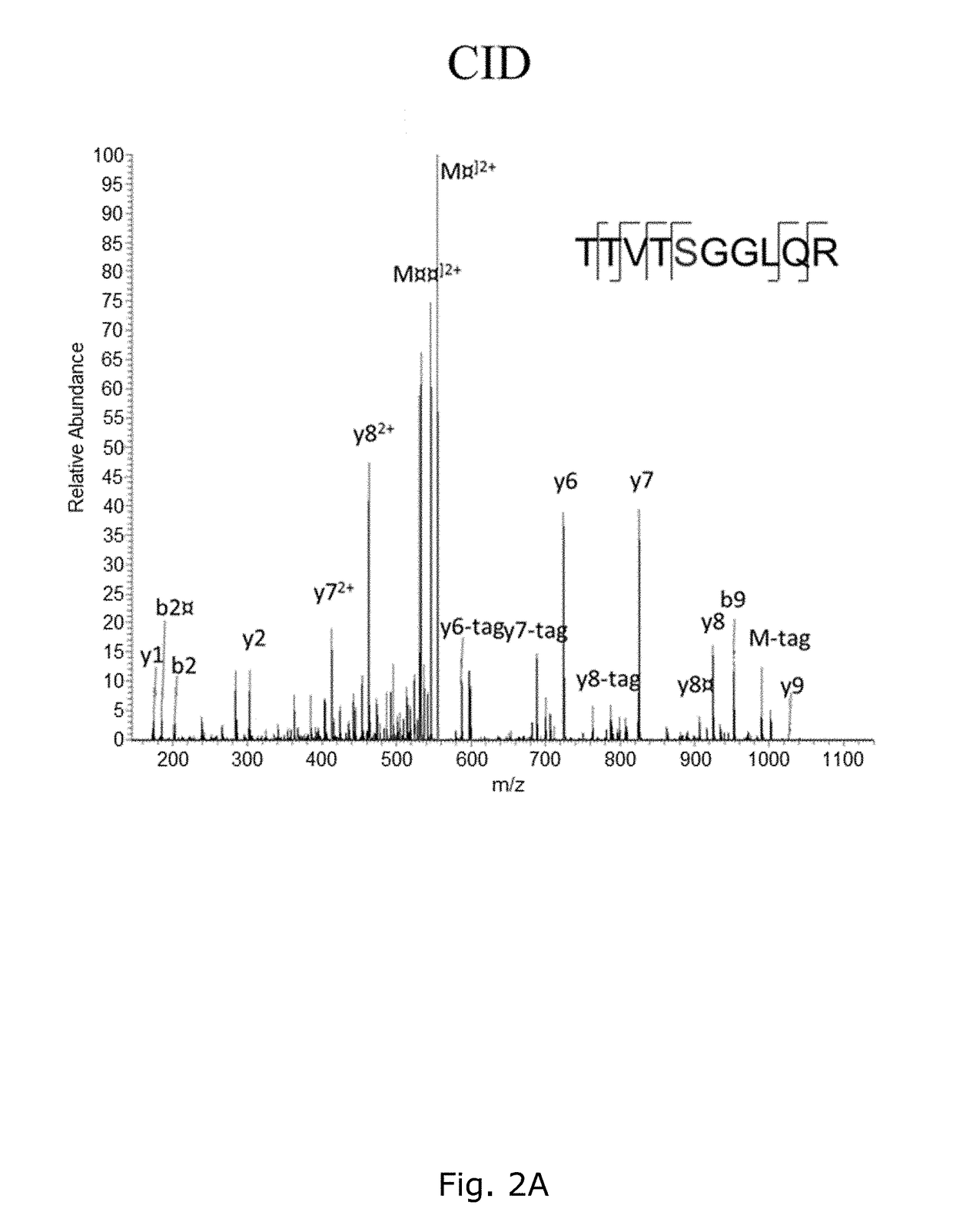
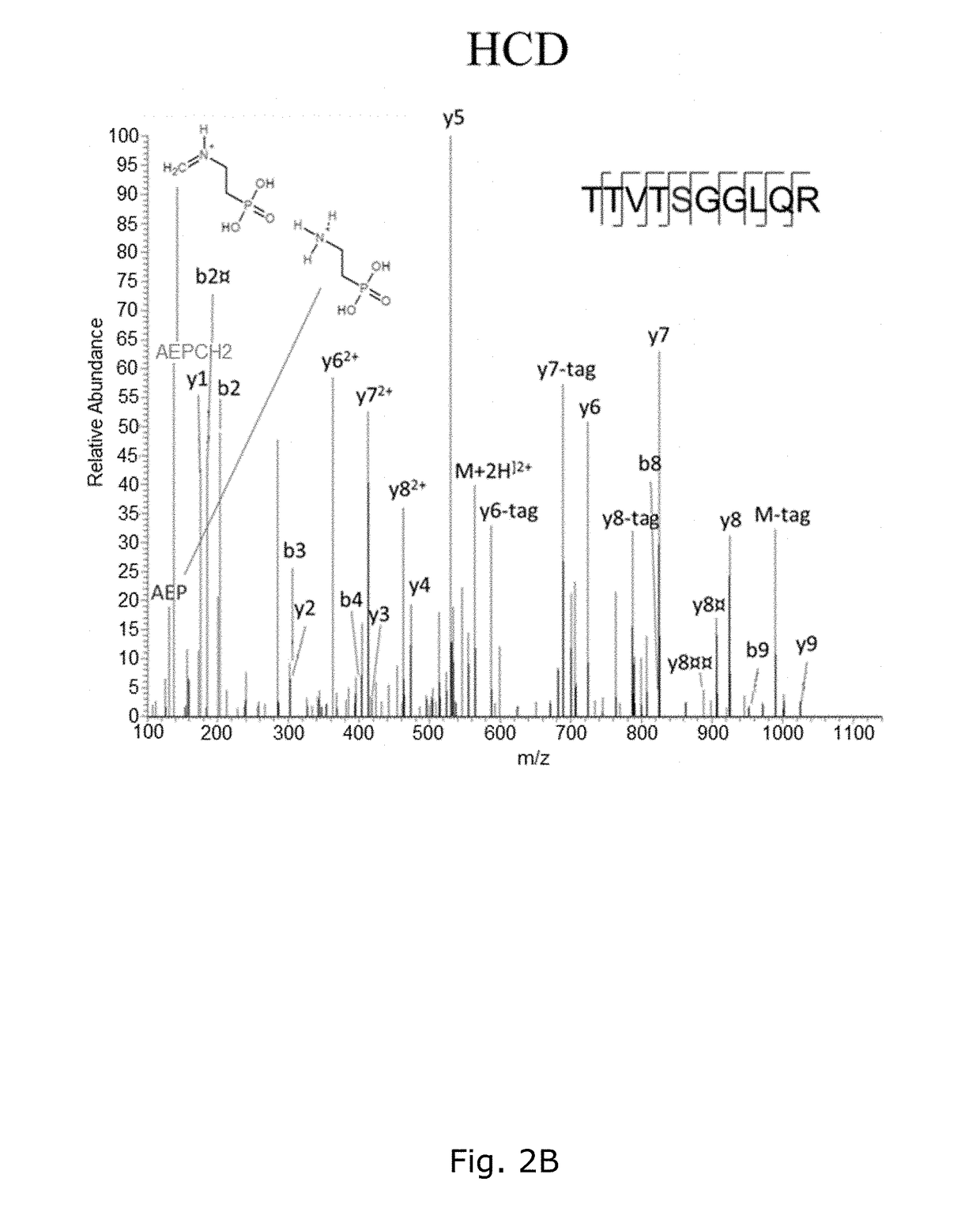
[0199]The BEMAP method was first established using a synthetic mannosylated peptide as a model compound. As shown in FIG. 1A and 1B, MALDI MS demonstrated that BEMAP efficiently replaces the carbohydrate moiety of the synthetic peptide (m / z=1181.59 Da) with the AEP group and thus produces a phosphopeptide (m / z=1126.64 Da).
[0200]The overall efficiency of substitution exceeds 95% (FIG. 1B) without the formation of degradation products. The AEP-modified peptide was then strongly enriched using affinity chroma...
example 2
Immunogenicity of ETEC Glycosylated Proteins
[0215]An immunogenic polypeptide is defined as a polypeptide that induces an immune response.
[0216]The immune response may be monitored by one of the following methods: An in vitro cellular response is determined by release of a relevant cytokine such as IFN-γ, from lymphocytes withdrawn from an animal or human currently or previously infected with ETEC, or by detection of proliferation of these T cells. The induction is performed by addition of the polypeptide or the immunogenic part to a suspension comprising from 1×105 cells to 3×105 cells per well. The cells are isolated from either blood, the spleen, the liver or the lung and the addition of the polypeptide or the immunogenic part of the polypeptide result in a concentration of not more than 20 μg per ml suspension and the stimulation is performed from two to five days. For monitoring cell proliferation, the cells are pulsed with radioactive labeled Thymidine and after 16-22 hours of ...
example 3
Schematic Overview of Assays and Experiments Used to Characterize Glycosylated as Well as Non-Glycosylated YghJ Protein Properties
[0224]
TABLE 2Type of experimentMouse challengeSerum and mucosal antibody responsesAntibody mediated inhibition of ETEC binding to Caco-2Antibody mediated inhibition of ETEC binding to Caco-2; cAMP releasemeasurementDegradation of intestinal mucin MUC3Quantitative YghJ - MUC3 interaction assessmentDegradation of intestinal mucin MUC2
[0225]An overview of the assays used for testing a wide variety of YghJ features is given in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com