Use of akkermansia for treating metabolic disorders
a metabolic disorder and akkermansia technology, applied in the field of metabolic disorders, can solve the problems of imbalance in the metabolism of carbohydrates, lipids, proteins and/or nucleic acids, and the beneficial effect of direct administration of akkermansia muciniphila has never been described, nor suggested, and achieves the effect of increasing the energy expenditure of said subjects, increasing the energy expenditure of subjects, and increasing the energy expenditur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0135]The present invention is further illustrated by the following examples.
[0136]Materials and Methods
[0137]Mice
[0138]Ob / Ob Experiment:
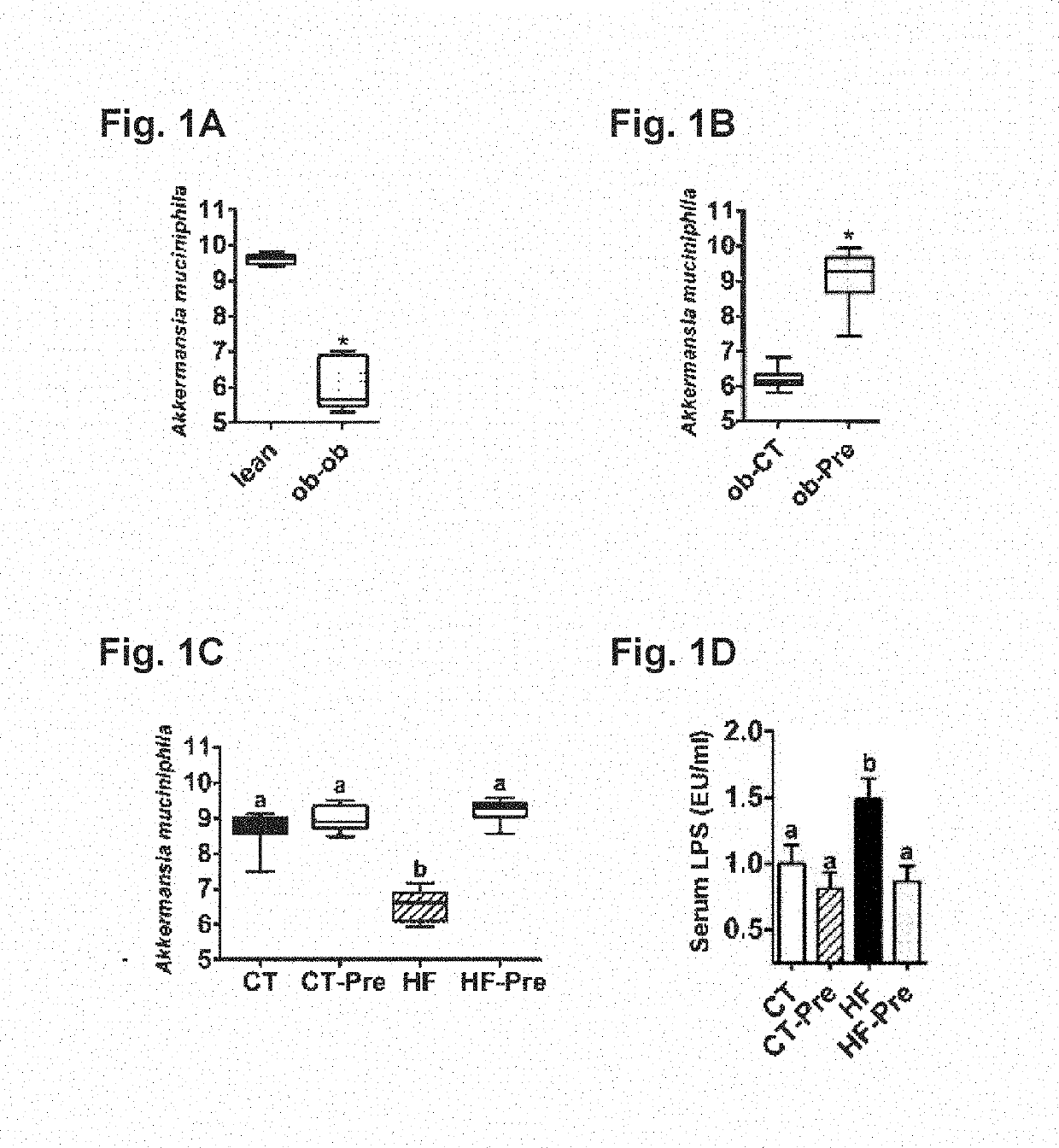
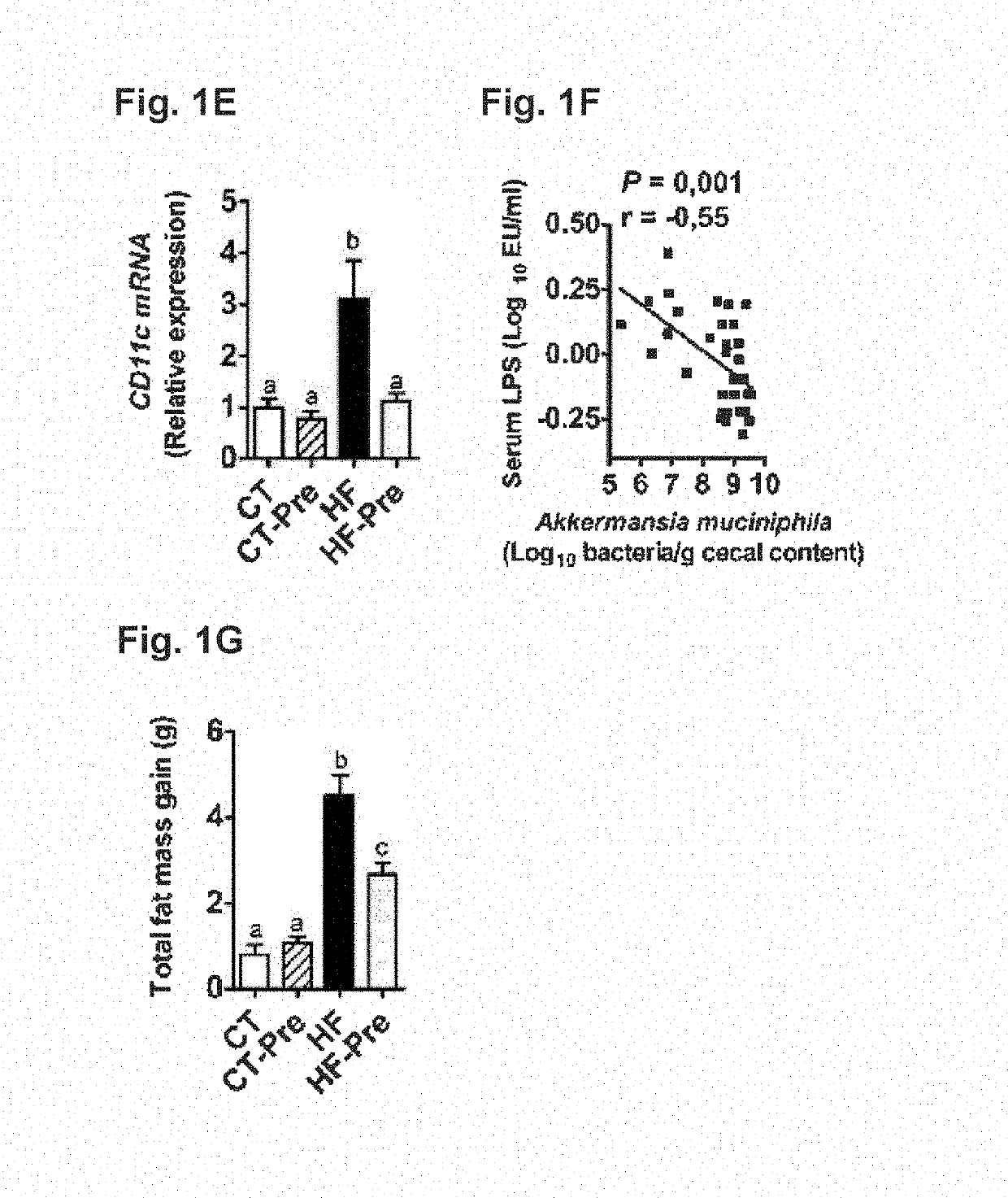
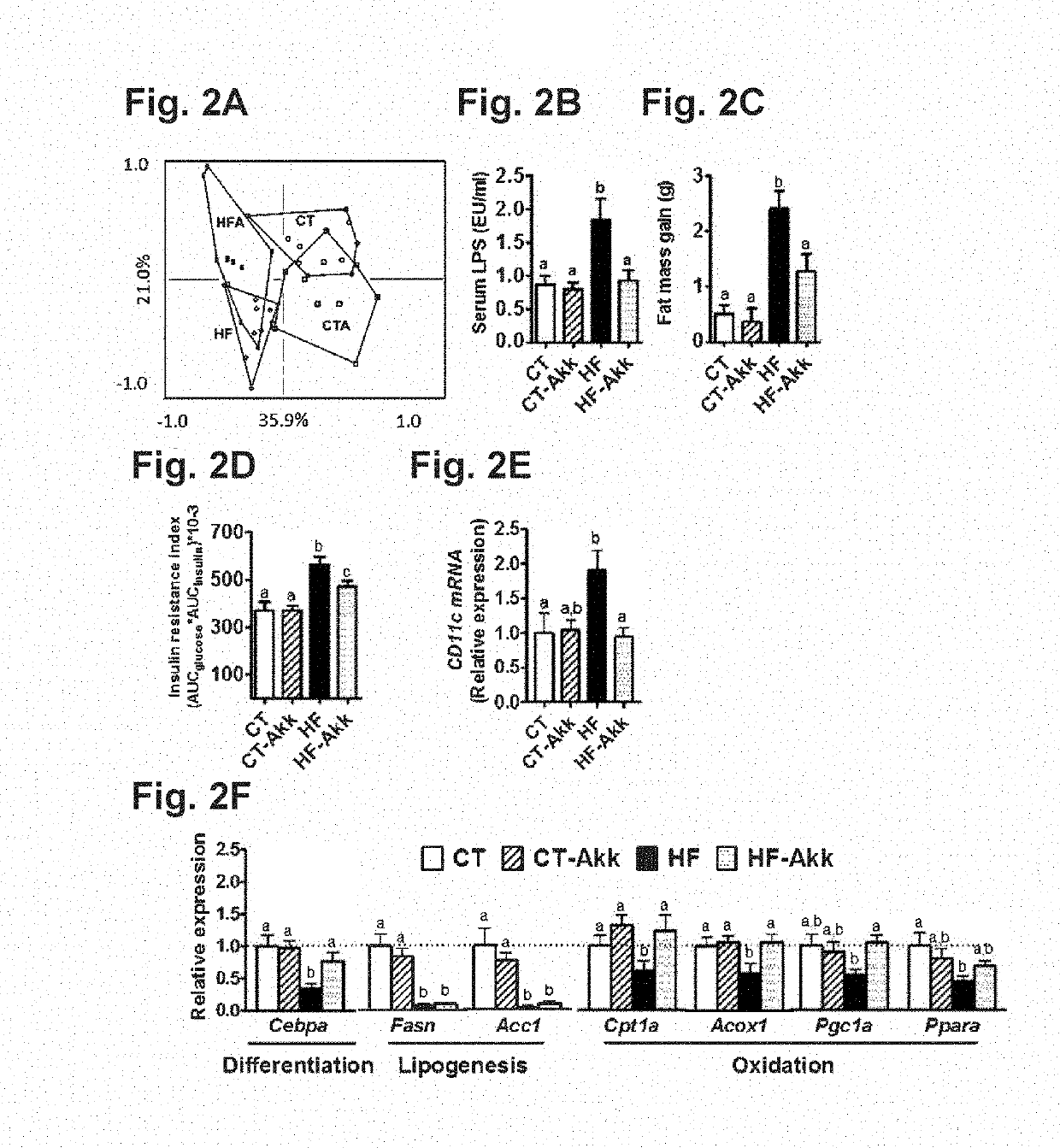
[0139]ob / ob versus lean study: Six-week-old ob / ob (n=5 / group) mice (C57BL / 6 background, Jackson-Laboratory, Bar Harbor, Me., USA) were housed in a controlled environment (12-h daylight cycle, lights-off at 6-pm) in groups of two or three mice / cage, with free access to food and water. The mice were fed a control diet (A04, Villemoisson-sur-Orge, France) for 16 weeks. Cecal content was harvested immersed in liquid nitrogen, and stored at −80° C., for further Akkermansia muciniphila analysis.
[0140]Ob / Ob Prebiotic Study:
[0141]Six-week-old ob / ob (n=10 / group) mice (C57BL / 6 background, Jackson-Laboratory, Bar Harbor, Me., USA) were housed in a controlled environment (12-h daylight cycle, lights-off at 6-pm) in groups of two mice / cage, with free access to food and water. The mice were fed a control diet (Ob-CT) (A04, Villemoisson-sur-Orge, France) or a con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com